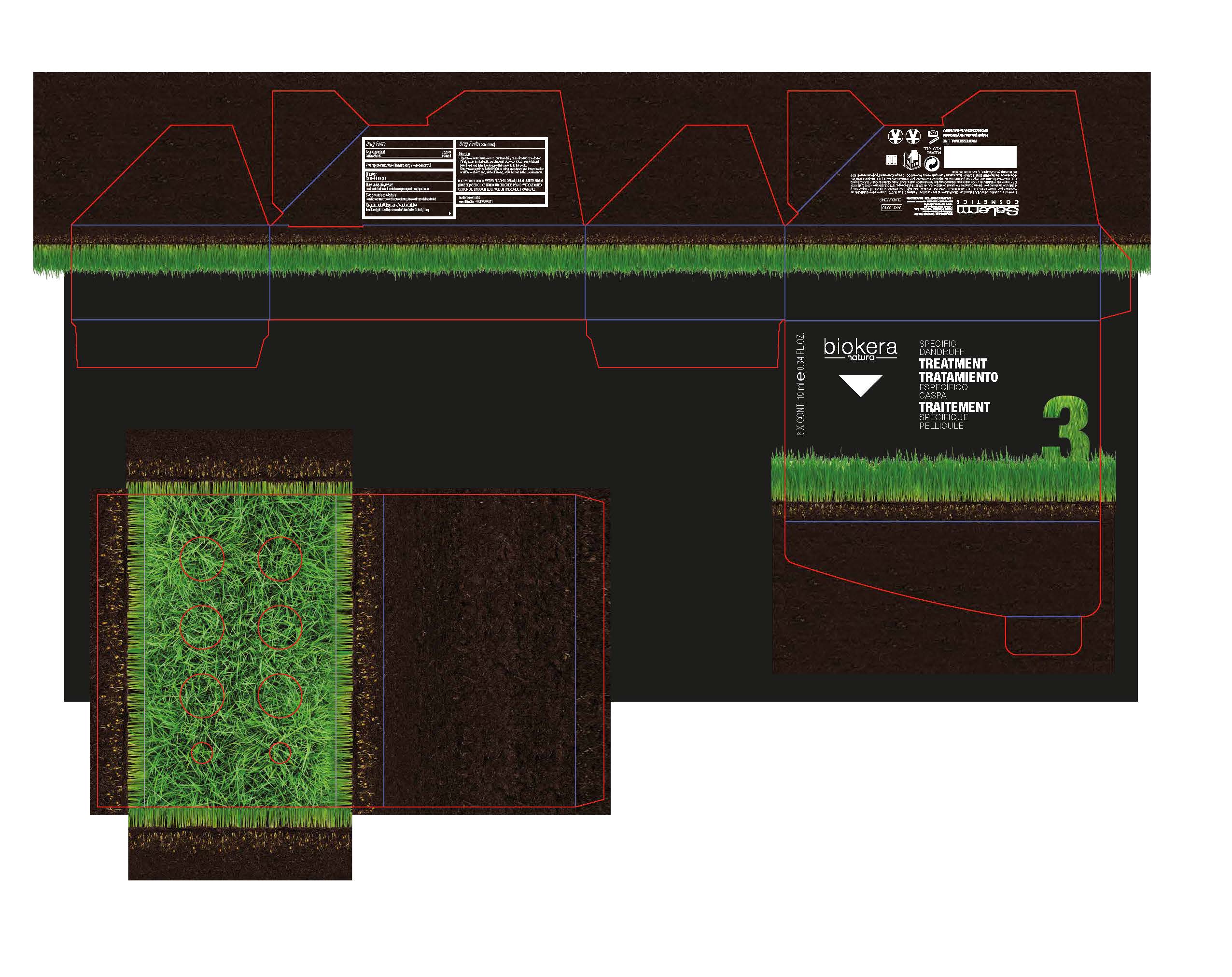
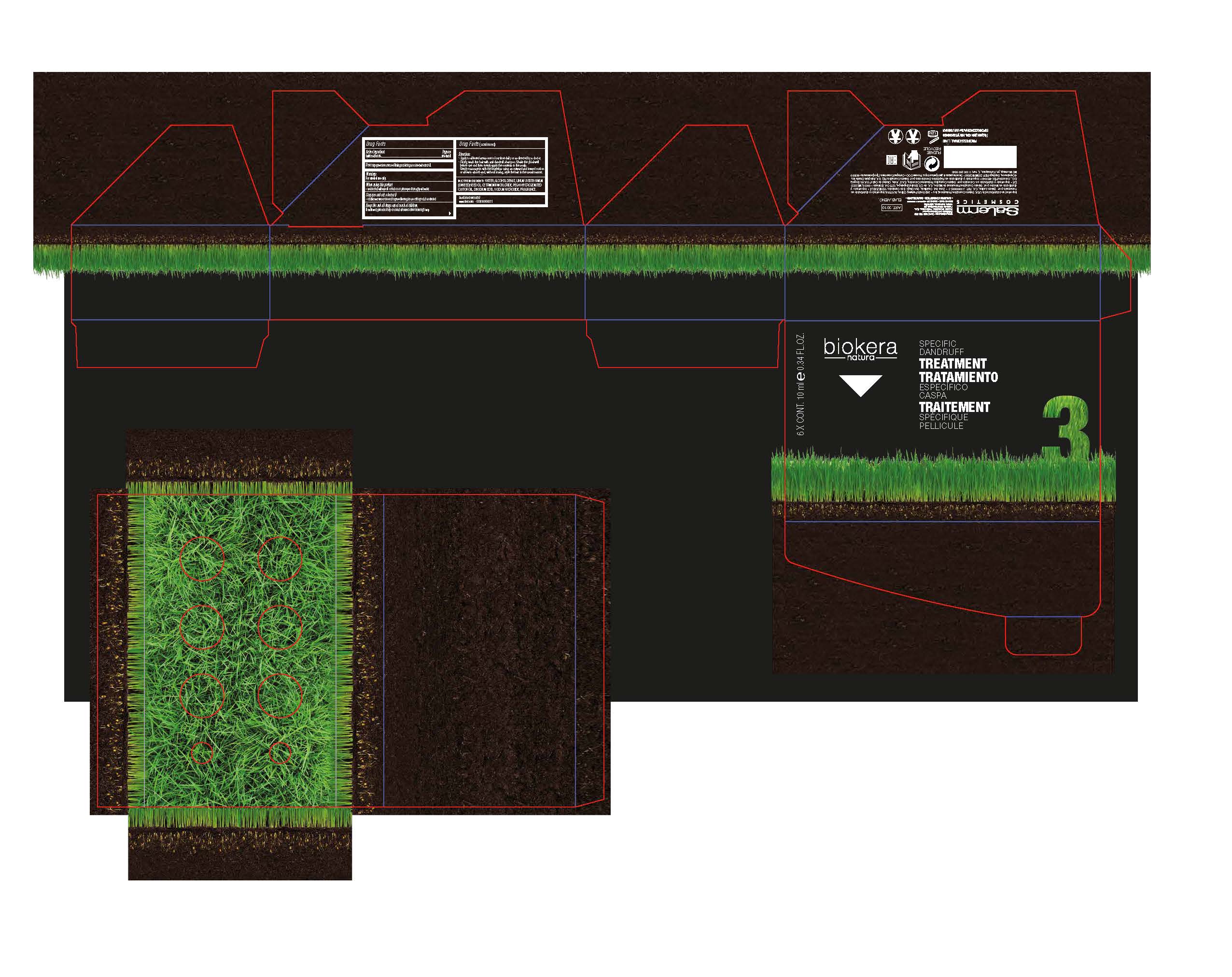
Label: BIOKERA- pyrithione zinc lotion/shampoo
-
Contains inactivated NDC Code(s)
NDC Code(s): 59538-003-03 - Packager: SALERM COSMETICA PROFESIONAL, S.A.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 2, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INACTIVE INGREDIENT
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- STOP USE
-
DOSAGE & ADMINISTRATION
Directions
-Apply to affected areas one to four time daily or as directed by a doctor.
-Firstly, wash the hair with anti-dandruff shampoo. Shake the phial well
before use and then evenly apply the contents to the scalp.
Gently massage in with the fingertips using an outward and inward motion
or allow to absorb and, without rinsing, style the hair in the usual manner.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BIOKERA
pyrithione zinc lotion/shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59538-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC .1 g in 10 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) FLAX SEED (UNII: 4110YT348C) LINSEED OIL (UNII: 84XB4DV00W) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59538-003-03 6 in 1 CARTON 08/01/2013 1 60 g in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 08/01/2013 Labeler - SALERM COSMETICA PROFESIONAL, S.A. (563418532)