Label: monistat 3- miconazole nitrate suppository
-
Contains inactivated NDC Code(s)
NDC Code(s): 0062-5437-01 - Packager: ORTHO PHARMACEUTICAL CORPORATION
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 31, 2006
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
-
DESCRIPTION
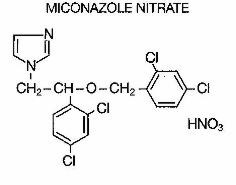
MONISTAT 3 Vaginal Suppositories are white to off-white suppositories, each containing the antifungal agent, miconazole nitrate, 1-[2,4-Dichloro-β-[(2,4-dichlorobenzyl)oxy]phenethyl]-imidazole mononitrate, 200 mg, in a hydrogenated vegetable oil base. Miconazole nitrate for vaginal use is also available as MONISTAT 7 Vaginal Cream and MONISTAT 7 Vaginal Suppositories.
-
CLINICAL PHARMACOLOGY
Miconazole nitrate exhibits fungicidal activity in vitro against species of the genus Candida. The pharmacologic mode of action is unknown. Following intravaginal administration of miconazole nitrate, small amounts are absorbed. Administration of a single dose of miconazole nitrate suppositories (100 mg) to healthy subjects resulted in a total recovery from the urine and feces of 0.85% (± 0.43%) of the administered dose.
Animal studies indicate that the drug crossed the placenta and doses above those used in humans result in embryo- and fetotoxicity (80 mg/kg, orally), although this has not been reported in human subjects (See PRECAUTIONS).
In multi-center clinical trials in 440 women with vulvovaginal candidiasis, the efficacy of treatment with the MONISTAT 3 Vaginal Suppository for 3 days was compared with treatment for 7 days with MONISTAT 7 Vaginal Cream. The clinical cure rates (free of microbiological evidence and clinical signs and symptoms of candidiasis at 8–10 days and 30–35 days post-therapy) were numerically lower, although statistically different, with the 3-Day Suppository when compared with the 7-Day Cream.
-
INDICATIONS AND USAGE
MONISTAT 3 Vaginal Suppositories are indicated for the local treatment of vulvovaginal candidiasis (moniliasis). Effectiveness in pregnancy and in diabetic patients has not been established. As MONISTAT is effective only for candidal vulvovaginitis, the diagnosis should be confirmed by KOH smear and/or cultures. Other pathogens commonly associated with vulvovaginitis (Trichomonas and Haemophilus vaginalis [Gardnerella]) should be ruled out by appropriate laboratory methods.
- CONTRAINDICATIONS
-
PRECAUTIONS
General
Discontinue drug if sensitization or irritation is reported during use. The base contained in the suppository formulation may interact with certain latex products, such as that used in vaginal contraceptive diaphragms. Concurrent use is not recommended. MONISTAT 7 Vaginal Cream may be considered for use under these conditions.
Laboratory Tests
If there is a lack of response to MONISTAT 3 Vaginal Suppositories, appropriate microbiological studies (standard KOH smear and/or cultures) should be repeated to confirm the diagnosis and rule out other pathogens.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies to determine carcinogenic potential have not been performed.
Fertility (Reproduction)
Oral administration of miconazole nitrate in rats has been reported to produce prolonged gestation. However, this effect was not observed in oral rabbit studies. In addition, signs of fetal and embryo toxicity were reported in rat and rabbit studies, and dystocia was reported in rat studies after oral doses at and above 80 mg per kg. Intravaginal administration did not produce these effects in rats.
Pregnancy
Since imidazoles are absorbed in small amounts from the human vagina, they should not be used in the first trimester of pregnancy unless the physician considers it essential to the welfare of the patient.
Clinical studies, during which miconazole nitrate vaginal cream and suppositories were used for up to 14 days, were reported to include 514 pregnant patients. Follow-up reports available in 471 of these patients reveal no adverse effects or complications attributable to miconazole nitrate therapy in infants born to these women.
-
ADVERSE REACTIONS
During clinical studies with MONISTAT 3 Vaginal Suppository (miconazole nitrate, 200 mg) 301 patients were treated. The incidence of vulvovaginal burning, itching or irritation was 2%. Complaints of cramping (2%) and headaches (1.3%) were also reported. Other complaints (hives, skin rash) occurred with less than a 0.5% incidence. The therapy-related dropout rate was 0.3%.
- OVERDOSE
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
INGREDIENTS AND APPEARANCE
MONISTAT 3
miconazole nitrate suppositoryProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0062-5437 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength miconazole nitrate (UNII: VW4H1CYW1K) (miconazole - UNII:7NNO0D7S5M) 200 mg Inactive Ingredients Ingredient Name Strength hydrogenated vegetable oil () Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0062-5437-01 3 in 1 PACKAGE Labeler - ORTHO PHARMACEUTICAL CORPORATION
