Label: BIOPROTECT HAND SANITIZER- benzalkonium chloride liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 73734-001-05, 73734-001-16, 73734-001-17, 73734-001-20, view more73734-001-24, 73734-001-25, 73734-001-28, 73734-001-30, 73734-001-34, 73734-001-55, 73734-001-57, 73734-001-80, 73734-001-96 - Packager: ViaClean Technologies, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 3, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- INACTIVE INGREDIENT
- Questions?
-
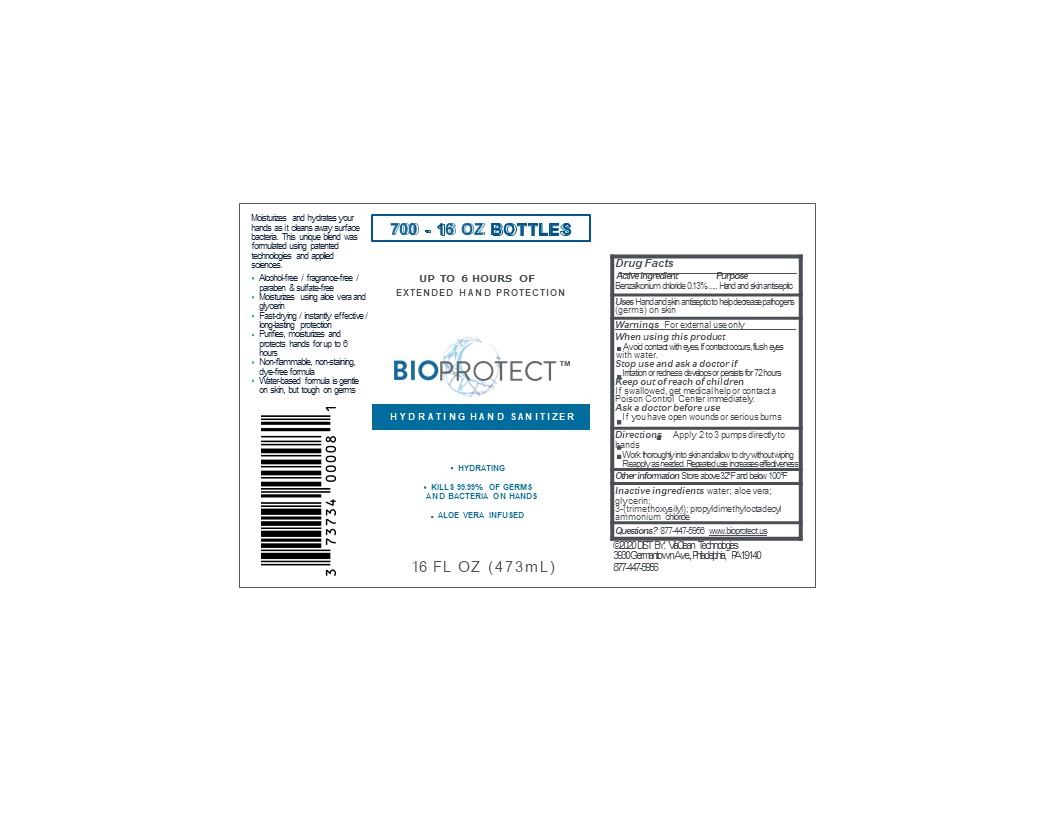
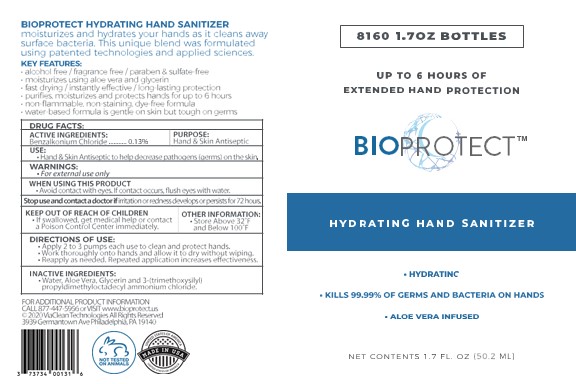
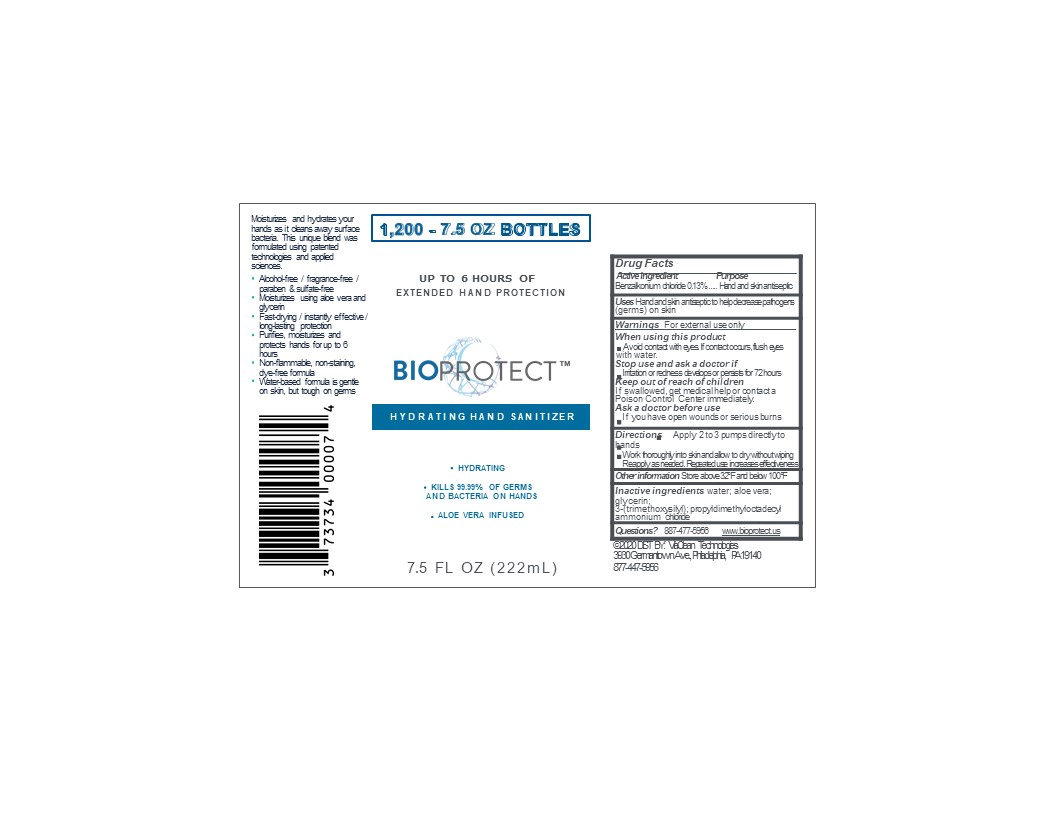
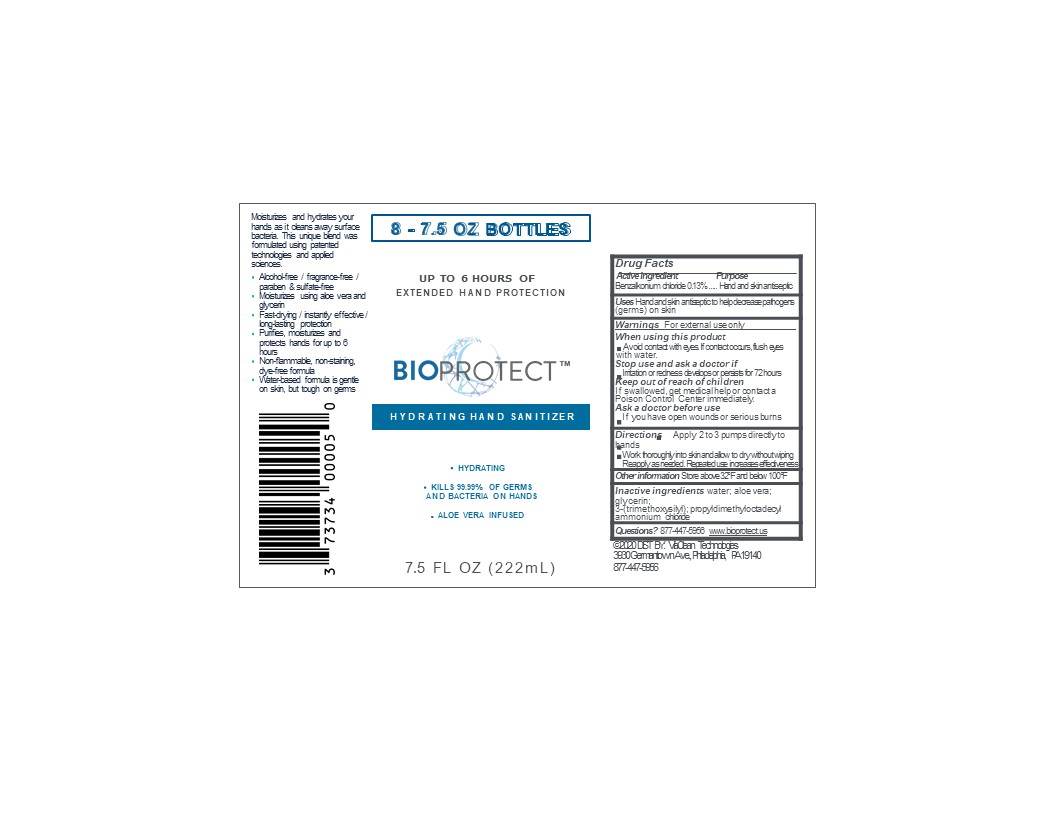
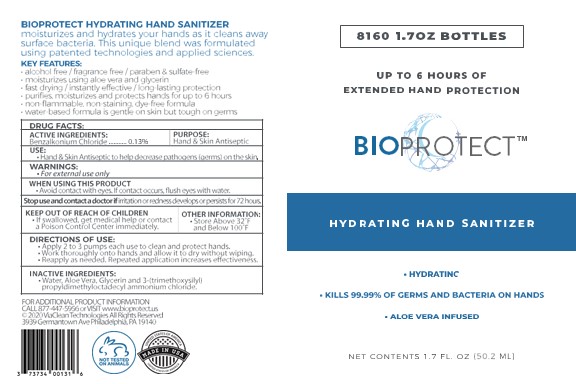
PRINCIPAL DISPLAY PANEL
BIOPROTECT
Hydrating hand sanitizer
Extended hand protection
Aloe Vera infused
Alcohol free/ fragrance free/ paraben and sulfate-free
Moisturizes using aloe vera and glycerin
Fast drying/instantly effective/ long-lasting protection
Non-flammable, non-staining, dye-free formula
Water-based formula is gentle on skin but tough on germs
Made in USA
(Manufactured and Distributed by)
ViaClean Technologies LLC
3939 Germantown Ave,Bldg. #2, Suite #2
Philadelphia, PA 19140
For additional product information: call 877-447-5956 or Visit www.bioprotect.us
(c) 2020 ViaClean Technologies All Rights Reserved
XXfl oz (XX mL) NDC 73734-001-XX
















-
INGREDIENTS AND APPEARANCE
BIOPROTECT HAND SANITIZER
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73734-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength DIMETHYLOCTADECYL(3-(TRIMETHOXYSILYL)PROPYL)AMMONIUM CHLORIDE (UNII: IQ36O85WQ4) WATER (UNII: 059QF0KO0R) ALOE (UNII: V5VD430YW9) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73734-001-34 100.5 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/19/2020 2 NDC:73734-001-80 236.6 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/19/2020 3 NDC:73734-001-16 473.1 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/19/2020 4 NDC:73734-001-20 591.4 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/19/2020 5 NDC:73734-001-28 3785 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/19/2020 6 NDC:73734-001-57 60 in 1 CASE 08/19/2020 6 NDC:73734-001-96 4 in 1 BOX 6 NDC:73734-001-24 24 in 1 CARTON 6 NDC:73734-001-17 50.2 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 7 NDC:73734-001-05 18927.1 mL in 1 PAIL; Type 0: Not a Combination Product 08/19/2020 8 NDC:73734-001-55 208198 mL in 1 DRUM; Type 0: Not a Combination Product 08/19/2020 9 NDC:73734-001-25 1040988.2406 mL in 1 CONTAINER, FLEXIBLE INTERMEDIATE BULK; Type 0: Not a Combination Product 08/19/2020 10 NDC:73734-001-30 50.2 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 08/19/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 08/19/2020 Labeler - ViaClean Technologies, LLC (108814788) Registrant - ViaClean Technologies, LLC (108814788) Establishment Name Address ID/FEI Business Operations Suite-K Value Added Service LLC 036822144 manufacture(73734-001)