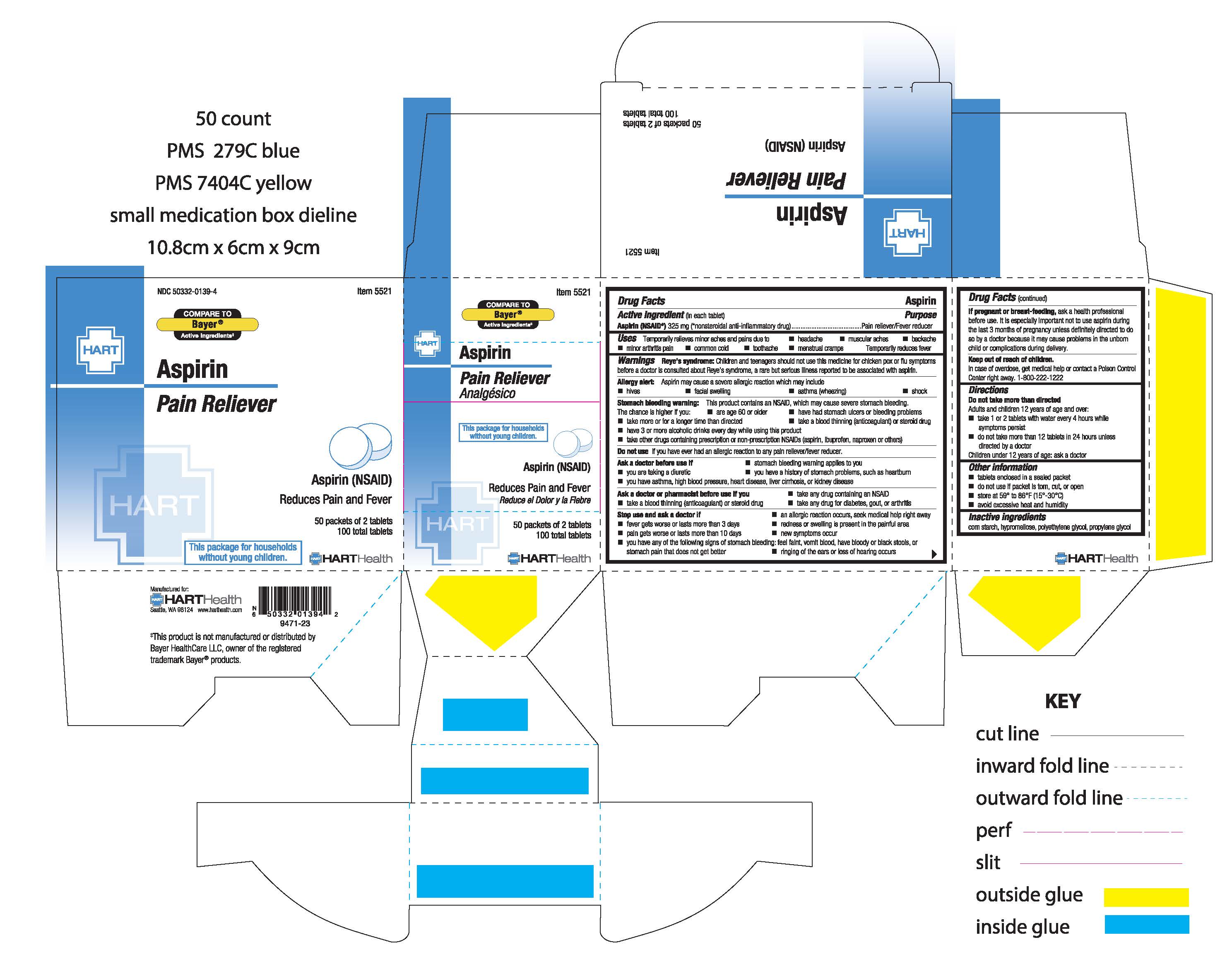
Label: ASPIRIN tablet
- NDC Code(s): 50332-0139-3, 50332-0139-4, 50332-0139-7, 50332-0139-8
- Packager: HART Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
Warnings
Reye's Syndrome: Children and teenagers should not use this medicine for chicken pox or flu symptoms before a doctor is consulted about Reye's syndrome, a rare but serious illness reported to be associated with aspirin
Allergy altert: Aspirin may cause a several allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulers or bleeding problems
- take more or for a longer time than directed
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or non-prescription NSAIDs (aspirin, ibuprofen, naproxen, others)
- have 3 or more alcholic drinks every day while using this product
- DO NOT USE
-
STOP USE
- an allergic reaction occurs, seek medical help right away
- fever gets worse or lasts more than 3 days
- pain gets worse or lasts more than 10 days
- redness or swelling is present in the painful area
- ringing in the ears or loss of hearing occurs
- new symptoms occur
- you have any of the following signs of stomach bleeding: feel faint, vomit blood, have bloody or black stools, or stomach pain that does not get better
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ASPIRIN
aspirin tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50332-0139 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color white Score no score Shape ROUND Size 10mm Flavor Imprint Code ASPIRIN;44;157 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50332-0139-4 50 in 1 BOX, UNIT-DOSE 01/01/2023 1 2 in 1 PACKET; Type 0: Not a Combination Product 2 NDC:50332-0139-7 125 in 1 BOX, UNIT-DOSE 01/01/2023 2 2 in 1 PACKET; Type 0: Not a Combination Product 3 NDC:50332-0139-8 250 in 1 BOX, UNIT-DOSE 01/01/2023 3 2 in 1 PACKET; Type 0: Not a Combination Product 4 NDC:50332-0139-3 25 in 1 BOX, UNIT-DOSE 01/01/2023 4 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M 01/01/2023 Labeler - HART Health (069560969)