Label: TECARTUS- brexucabtagene autoleucel suspension
- NDC Code(s): 71287-219-01, 71287-219-02, 71287-220-01, 71287-220-02
- Packager: Kite Pharma, Inc.
- Category: CELLULAR THERAPY
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated October 21, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TECARTUS safely and effectively. See full prescribing information for TECARTUS.
TECARTUS® (brexucabtagene autoleucel) suspension for intravenous infusion
Initial U.S. Approval: 2020WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, AND SECONDARY HEMATOLOGICAL MALIGNANCIES
See full prescribing information for complete boxed warning.
- Cytokine Release Syndrome (CRS), including life-threatening reactions, occurred in patients receiving TECARTUS. Do not administer TECARTUS to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids (2.2, 2.3, 5.1).
- Neurologic toxicities, including life-threatening reactions, occurred in patients receiving TECARTUS, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with TECARTUS. Provide supportive care and/or corticosteroids, as needed (2.2, 2.3, 5.2).
- T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies (5.8).
RECENT MAJOR CHANGES
Boxed Warning 04/2024 Dosage and Administration (2.2, 2.3) 06/2025 Warnings and Precautions (5.1, 5.2) 06/2025 Warnings and Precautions, YESCARTA and TECARTUS REMS (5.3) Removed 06/2025 Warnings and Precautions, Secondary Malignancies (5.8) 04/2024 Warnings and Precautions, Effects on Ability to Drive and Use Machines (5.10) Removed 06/2025 INDICATIONS AND USAGE
TECARTUS is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of: (1)
- Adult patients with relapsed or refractory mantle cell lymphoma (MCL).
This indication is approved under accelerated approval based on overall response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. - Adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
DOSAGE AND ADMINISTRATION
For autologous use only. For intravenous use only.
- Do NOT use a leukodepleting filter.
- Administer a lymphodepleting regimen of cyclophosphamide and fludarabine before infusion of TECARTUS. (2.2)
- Verify the patient's identity prior to infusion. (2.2)
- Premedicate with acetaminophen and diphenhydramine. (2.2)
- Confirm availability of tocilizumab prior to infusion. (2.2, 5.1)
- Dosing of TECARTUS is based on the number of chimeric antigen receptor (CAR)-positive viable T cells. (2.1)
- MCL: dose is 2 × 106 CAR-positive viable T cells per kg body weight, with a maximum of 2 × 108 CAR-positive viable T cells. (2.1)
- ALL: dose is 1 × 106 CAR-positive viable T cells per kg body weight, with a maximum of 1 × 108 CAR-positive viable T cells. (2.1)
DOSAGE FORMS AND STRENGTHS
- TECARTUS is available as a cell suspension for infusion.
- MCL: Comprises a suspension of 2 × 106 CAR-positive viable T cells per kg of body weight, with a maximum of 2 × 108 CAR-positive viable T cells in approximately 68 mL. (3)
- ALL: Comprises a suspension of 1 × 106 CAR-positive viable T cells per kg of body weight, with a maximum of 1 × 108 CAR-positive viable T cells in approximately 68 mL. (3)
CONTRAINDICATIONS
- None. (4)
WARNINGS AND PRECAUTIONS
- Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome: Administer treatment per institutional standards. (5.3)
- Hypersensitivity Reactions: Monitor for hypersensitivity reactions during infusion. (5.4)
- Severe Infections: Monitor patients for signs and symptoms of infection; treat appropriately. (5.5)
- Prolonged Cytopenias: Patients may exhibit Grade 3 or higher cytopenias for several weeks following TECARTUS infusion. Monitor complete blood counts. (5.6)
- Hypogammaglobulinemia: Monitor and provide replacement therapy. (5.7)
- Secondary Malignancies: T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies. In the event that a secondary malignancy occurs after treatment with TECARTUS, contact Kite at 1-844-454-KITE (5483). (5.8)
ADVERSE REACTIONS
The most common non-laboratory adverse reactions (incidence greater than or equal to 20%) are:
- MCL: fever, CRS, hypotension, encephalopathy, fatigue, tachycardia, arrhythmia, infection with pathogen unspecified, chills, hypoxia, cough, tremor, musculoskeletal pain, headache, nausea, edema, motor dysfunction, constipation, diarrhea, decreased appetite, dyspnea, rash, insomnia, pleural effusion, and aphasia. (6.1)
- ALL: fever, CRS, hypotension, encephalopathy, tachycardia, nausea, chills, headache, fatigue, febrile neutropenia, diarrhea, musculoskeletal pain, hypoxia, rash, edema, tremor, infection with pathogen unspecified, constipation, decreased appetite, and vomiting. (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Kite at 1-844-454-KITE (5483) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, and SECONDARY HEMATOLOGICAL MALIGNANCIES
1 INDICATIONS AND USAGE
1.1 Mantle Cell Lymphoma
1.2 Acute Lymphoblastic Leukemia
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Administration
2.3 Management of Severe Adverse Reactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome
5.2 Neurologic Toxicities
5.3 Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome
5.4 Hypersensitivity Reactions
5.5 Severe Infections
5.6 Prolonged Cytopenias
5.7 Hypogammaglobulinemia
5.8 Secondary Malignancies
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Relapsed or Refractory Mantle Cell Lymphoma
14.2 Relapsed or Refractory B-cell precursor Acute Lymphoblastic Leukemia
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, and SECONDARY HEMATOLOGICAL MALIGNANCIES
- Cytokine Release Syndrome (CRS), including life-threatening reactions, occurred in patients receiving TECARTUS. Do not administer TECARTUS to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.1)].
- Neurologic toxicities, including life-threatening reactions, occurred in patients receiving TECARTUS, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with TECARTUS. Provide supportive care and/or corticosteroids, as needed [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.2)].
- T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies [see Warnings and Precautions (5.8)].
-
1 INDICATIONS AND USAGE
TECARTUS is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of:
1.1 Mantle Cell Lymphoma
Adult patients with relapsed or refractory mantle cell lymphoma (MCL).
This indication is approved under accelerated approval based on overall response rate and durability of response [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
-
2 DOSAGE AND ADMINISTRATION
For autologous use only. For intravenous use only.
Each single infusion bag of TECARTUS contains a suspension of chimeric antigen receptor (CAR)-positive T cells in approximately 68 mL.
2.1 Dose
2.2 Administration
TECARTUS is for autologous use only. The patient's identity must match the patient identifiers on the TECARTUS cassette and infusion bag. Do not infuse TECARTUS if the information on the patient-specific label does not match the intended patient.
Preparing Patient for TECARTUS Infusion
Confirm availability of TECARTUS prior to starting the lymphodepleting chemotherapy regimen.
Pre-treatment
- MCL: Administer a lymphodepleting chemotherapy regimen of cyclophosphamide 500 mg/m2 intravenously and fludarabine 30 mg/m2 intravenously on each of the fifth, fourth, and third day before infusion of TECARTUS.
- ALL: Administer a lymphodepleting chemotherapy regimen of fludarabine 25 mg/m2 intravenously over 30 minutes on the fourth, third, and second day and administer cyclophosphamide 900 mg/m2 over 60 minutes on the second day before infusion of TECARTUS.
Preparation of TECARTUS for infusion
Coordinate the timing of TECARTUS thaw and infusion. Confirm the infusion time in advance, and adjust the start time of TECARTUS thaw such that TECARTUS will be available for infusion when the patient is ready.
- Confirm patient identity: Prior to TECARTUS preparation, match the patient's identity with the patient identifiers on the TECARTUS cassette.
- Do not remove the TECARTUS infusion bag from the cassette if the patient information on the cassette label does not match the intended patient.
- Once patient identity is confirmed, remove the TECARTUS infusion bag from the cassette and check that the patient information on the cassette label matches the patient information on the bag label.
- Inspect the infusion bag for any breaches of container integrity such as breaks or cracks before thawing. If the bag is compromised, follow the local guidelines (or call Kite at 1-844-454-KITE).
- Place the infusion bag inside a second sterile bag per local guidelines.
- Thaw the infusion bag at approximately 37°C using either a water bath or dry-thaw method until there is no visible ice in the infusion bag.
- Gently mix the contents of the bag to disperse clumps of cellular material. If visible cell clumps remain, continue to gently mix the contents of the bag. Small clumps of cellular material should disperse with gentle manual mixing. Do not wash, spin down, and/or re-suspend TECARTUS in new media prior to infusion.
- Once thawed, TECARTUS should be administered within 30 minutes but may be stored at room temperature (20°C to 25°C) for up to three hours.
Administration
- For autologous use only.
- Confirm that tocilizumab and emergency equipment are available prior to infusion and during the recovery period.
- Do NOT use a leukodepleting filter.
- Central venous access is recommended for the administration of TECARTUS.
- Confirm that the patient's identity matches the patient identifiers on the TECARTUS infusion bag.
- Prime the tubing with normal saline prior to infusion.
- Infuse the entire contents of the TECARTUS bag within 30 minutes by either gravity or a peristaltic pump. TECARTUS is stable at room temperature for up to three hours after thaw.
- Gently agitate the TECARTUS bag during infusion to prevent cell clumping.
- After the entire contents of the TECARTUS bag are infused, rinse the tubing with normal saline at the same infusion rate to ensure all product is delivered.
TECARTUS contains human blood cells that are genetically modified with replication-incompetent retroviral vector. Follow universal precautions and local biosafety guidelines for handling and disposal of TECARTUS to avoid potential transmission of infectious diseases.
Monitoring
- Monitor patients daily for at least 7 days following infusion for signs and symptoms of Cytokine Release Syndrome (CRS) and neurologic events.
- Instruct patients to remain within proximity of a healthcare facility for at least 2 weeks following infusion.
- Advise patients to avoid driving for at least 2 weeks following infusion.
2.3 Management of Severe Adverse Reactions
Cytokine Release Syndrome (CRS)
Identify CRS based on clinical presentation [see Warnings and Precautions (5.1)]. Evaluate for and treat other causes of fever, hypoxia, and hypotension. If CRS is suspected, manage according to the recommendations in Table 1. Patients who experience Grade 2 or higher CRS (e.g., hypotension, not responsive to fluids, or hypoxia requiring supplemental oxygenation) should be monitored with continuous cardiac telemetry and pulse oximetry. For patients experiencing severe CRS, consider performing an echocardiogram to assess cardiac function. For severe or life-threatening CRS, consider intensive care supportive therapy. Physicians may also consider management per current practice guidelines.
Table 1. CRS Grading and Management Guidance CRS Grade* Tocilizumab Corticosteroids Grade 1
Symptoms require symptomatic treatment only (e.g., fever, nausea, fatigue, headache, myalgia, malaise).If not improving after 24 hours, administer tocilizumab† 8 mg/kg intravenously over 1 hour (not to exceed 800 mg). Not applicable. Grade 2
Symptoms require and respond to moderate intervention.
Oxygen requirement less than 40% FiO2 or hypotension responsive to fluids or low dose of one vasopressor or Grade 2 organ toxicity.‡Administer tocilizumab 8 mg/kg intravenously over 1 hour (not to exceed 800 mg).
Repeat tocilizumab every 8 hours as needed if not responsive to intravenous fluids or increasing supplemental oxygen. Limit to a maximum of 3 doses in a 24-hour period; maximum total of 4 doses if no clinical improvement in the signs and symptoms of CRS.
If improving, discontinue tocilizumab.Manage per Grade 3 if no improvement within 24 hours after starting tocilizumab.
If improving, taper corticosteroids.Grade 3
Symptoms require and respond to aggressive intervention.
Oxygen requirement greater than or equal to 40% FiO2 or hypotension requiring high-dose or multiple vasopressors or Grade 3 organ toxicity or Grade 4 transaminitis.Per Grade 2
If improving, discontinue tocilizumab.Administer methylprednisolone 1 mg/kg intravenously twice daily or equivalent dexamethasone (e.g., 10 mg intravenously every 6 hours) until Grade 1, then taper corticosteroids.
If improving, manage as Grade 2.
If not improving, manage as Grade 4.Grade 4
Life-threatening symptoms.
Requirements for ventilator support or continuous veno-venous hemodialysis (CVVHD), or Grade 4 organ toxicity (excluding transaminitis).Per Grade 2
If improving, discontinue tocilizumab.Administer methylprednisolone 1000 mg intravenously per day for 3 days.
If improving, taper corticosteroids, and manage as Grade 3.
If not improving, consider alternate immunosuppressants.Neurologic Toxicity
Monitor patients for signs and symptoms of neurologic toxicities/immune effector cell-associated neurotoxicity syndrome (ICANS) (Table 2). Rule out other causes of neurologic symptoms. Patients who experience Grade 2 or higher neurologic toxicities/ICANS should be monitored with continuous cardiac telemetry and pulse oximetry. Provide intensive care supportive therapy for severe or life-threatening neurologic toxicities. Consider non-sedating anti-seizure medicines (e.g., levetiracetam) for seizure prophylaxis for any Grade 2 or higher neurologic toxicities. Physicians may also consider management per current practice guidelines.
Table 2. Neurologic Toxicity/ICANS Grading and Management Guidance Neurologic Event* Concurrent CRS No Concurrent CRS Abbreviation: ADLs, activities of daily living. - *
- Severity based on Common Terminology Criteria for Adverse Events.
Grade 1
Examples include:
Somnolence – mild drowsiness or sleepiness
Confusion – mild disorientation
Encephalopathy – mild limiting of ADLs
Dysphasia – not impairing ability to communicateAdminister tocilizumab per Table 1 for management of Grade 1 CRS. Supportive care. Grade 2
Examples include:
Somnolence – moderate limiting instrumental ADLs
Confusion – moderate disorientation
Encephalopathy – limiting instrumental ADLs
Dysphasia moderate impairing ability to communicate spontaneously
Seizure(s)Administer tocilizumab per Table 1 for management of Grade 2 CRS.
If not improving within 24 hours after starting tocilizumab, administer dexamethasone 10 mg intravenously every 6 hours until the event is Grade 1 or less, then taper corticosteroids.
If improving, discontinue tocilizumab.
If still not improving, manage as Grade 3.Administer dexamethasone 10 mg intravenously every 6 hours until the event is Grade 1 or less.
If improving, taper corticosteroids.Consider non-sedating, anti-seizure medicines (e.g., levetiracetam) for seizure prophylaxis. Grade 3
Examples include:
Somnolence – obtundation or stupor
Confusion – severe disorientation
Encephalopathy – limiting self-care ADLs
Dysphasia – severe receptive or expressive characteristics, impairing ability to read, write, or communicate intelligiblyAdminister tocilizumab per Table 1 for management of Grade 2 CRS.
In addition, administer dexamethasone 10 mg intravenously with the first dose of tocilizumab and repeat dose every 6 hours. Continue dexamethasone use until the event is Grade 1 or less, then taper corticosteroids.
If improving, discontinue tocilizumab and manage as Grade 2.
If still not improving, manage as Grade 4.Administer dexamethasone 10 mg intravenously every 6 hours.
Continue dexamethasone use until the event is Grade 1 or less, then taper corticosteroids.
If not improving, manage as Grade 4.Consider non-sedating anti-seizure medicines (e.g., levetiracetam) for seizure prophylaxis. Grade 4
Life-threatening consequences
Urgent intervention indicated
Requirement for mechanical ventilation
Consider cerebral edemaAdminister tocilizumab per Table 1 for management of Grade 2 CRS.
Administer methylprednisolone 1000 mg intravenously per day with first dose of tocilizumab and continue methylprednisolone 1000 mg intravenously per day for 2 more days.
If improving, then manage as Grade 3.
If not improving, consider alternate immunosuppressants.Administer methylprednisolone 1000 mg intravenously per day for 3 days.
If improving, then manage as Grade 3.
If not improving, consider alternate immunosuppressants.Consider non-sedating anti-seizure medicines (e.g., levetiracetam) for seizure prophylaxis. -
3 DOSAGE FORMS AND STRENGTHS
TECARTUS is available as a cell suspension for infusion.
- MCL: A single dose of TECARTUS contains 2 × 106 CAR-positive viable T cells per kg of body weight [maximum of 2 × 108 CAR-positive viable T cells (for patients 100 kg and above)] in approximately 68 mL suspension in an infusion bag [see How Supplied/Storage and Handling (16)].
- ALL: A single dose of TECARTUS contains 1 × 106 CAR-positive viable T cells per kg of body weight [maximum of 1 × 108 CAR-positive viable T cells (for patients 100 kg and above)] in approximately 68 mL suspension in an infusion bag [see How Supplied/Storage and Handling (16)].
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome
CRS, including fatal or life-threatening reactions, occurred following treatment with TECARTUS. CRS occurred in 91% (75/82) of patients with MCL, including ≥ Grade 3 (Lee grading system1) CRS in 18% of patients. Among the patients with MCL who died after receiving TECARTUS, one patient had a fatal CRS event. The median time to onset of CRS was three days (range: 1 to 13 days) and the median duration of CRS was ten days (range: 1 to 50 days) for patients with MCL. CRS occurred in 92% (72/78) of patients with ALL, including ≥ Grade 3 (Lee grading system1) CRS in 26% of patients. Three patients with ALL had ongoing CRS events at the time of death. The median time to onset of CRS was five days (range: 1 to 12 days) and the median duration of CRS was eight days (range: 2 to 63 days) for patients with ALL.
The incidence of CRS (first occurrence) within the first 7 days after TECARTUS infusion was 83% (68/82) in patients with MCL and 90% (70/78) in patients with ALL. In all patients combined (MCL/ALL), the incidence of first CRS (first occurrence) within the first 7 days after TECARTUS infusion was 86% (138/160).
Among patients with CRS, the key manifestations (>10%) were similar in MCL and ALL and included fever (93%), hypotension (62%), tachycardia (59%), chills (32%), hypoxia (31%), headache (21%), fatigue (20%), and nausea (13%). Serious events associated with CRS in MCL and ALL combined (≥ 2%) included hypotension, fever, hypoxia, tachycardia, and dyspnea [see Adverse Reactions (6)].
Confirm that a minimum of two doses of tocilizumab are available for each patient prior to infusion of TECARTUS. Monitor patients daily for at least 7 days following infusion for signs and symptoms of CRS. Monitor patients for signs or symptoms of CRS for 2 weeks after infusion. Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time [see Patient Counseling Information (17)]. At the first sign of CRS, institute treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids as indicated [see Dosage and Administration (2.3)].
5.2 Neurologic Toxicities
Neurologic toxicities (including ICANS) that were fatal or life-threatening, occurred following treatment with TECARTUS. Neurologic events occurred in 81% (66/82) of patients with MCL, including ≥ Grade 3 in 37% of patients. The median time to onset for neurologic events was six days (range: 1 to 32 days) with a median duration of 21 days (range: 2 to 454 days) in patients with MCL. Neurologic events occurred in 87% (68/78) of patients with ALL, including ≥ Grade 3 in 35% of patients. The median time to onset for neurologic events was 7 days (range: 1 to 51 days) with a median duration of 15 days (range: 1 to 397 days) in patients with ALL. For patients with MCL, 54 (66%) patients experienced CRS before the onset of neurological events. Five (6%) patients did not experience CRS with neurologic events and eight patients (10%) developed neurological events after the resolution of CRS. Neurologic events resolved for 119 out of 134 (89%) patients treated with TECARTUS. Nine patients (three patients with MCL and six patients with ALL) had ongoing neurologic events at the time of death. For patients with ALL, neurologic events occurred before, during, and after CRS in 4 (5%), 57 (73%), and 8 (10%) of patients; respectively. Three patients (4%) had neurologic events without CRS. The onset of neurologic events can be concurrent with CRS, following resolution of CRS or in the absence of CRS.
The incidence of neurologic events (first occurrence) within the first 7 days after TECARTUS infusion was 56% (46/82) in patients with MCL and 55% (43/78) in patients with ALL. In all patients combined (MCL/ALL) the incidence of neurologic events (first occurrence) within the first 7 days after TECARTUS infusion was 56% (89/160). Ninety-one percent of all treated patients experienced the first CRS or neurological event within the first 7 days after TECARTUS infusion.
The most common neurologic events (>10%) were similar in MCL and ALL and included encephalopathy (57%), headache (37%), tremor (34%), confusional state (26%), aphasia (23%), delirium (17%), dizziness (15%), anxiety (14%), and agitation (12%). Serious events (≥ 2%) including encephalopathy, aphasia, confusional state, and seizures occurred after treatment with TECARTUS.
Monitor patients daily for at least 7 days following infusion for signs and symptoms of neurologic toxicity/ICANS. Monitor patients for signs or symptoms of neurologic toxicities for 2 weeks after infusion and treat promptly [see Dosage and Administration (2.3)]. Advise patients to avoid driving for at least 2 weeks following infusion.
5.3 Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome
Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome (HLH/MAS), including life-threatening reactions, occurred following treatment with TECARTUS. HLH/MAS occurred in 4% (3/78) of patients with ALL. Two patients experienced Grade 3 events and 1 patient experienced a Grade 4 event. The median time to onset for HLH/MAS was 8 days (range: 6 to 9 days) with a median duration of 5 days (range: 2 to 8 days). All three patients with HLH/MAS had concurrent CRS symptoms and neurologic events after TECARTUS infusion. Treatment of HLH/MAS should be administered per institutional standards.
5.4 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, may occur due to dimethyl sulfoxide (DMSO) or residual gentamicin in TECARTUS.
5.5 Severe Infections
Severe or life-threatening infections occurred in patients after TECARTUS infusion. Infections (all grades) occurred in 56% (46/82) of patients with MCL and 44% (34/78) of patients with ALL. Grade 3 or higher infections, including bacterial, viral, and fungal infections, occurred in 30% of patients with ALL and MCL. TECARTUS should not be administered to patients with clinically significant active systemic infections. Monitor patients for signs and symptoms of infection before and after TECARTUS infusion and treat appropriately. Administer prophylactic antimicrobials according to local guidelines.
Febrile neutropenia was observed in 6% of patients with MCL and 35% of patients with ALL after TECARTUS infusion and may be concurrent with CRS. The febrile neutropenia in 27 (35%) of patients with ALL includes events of "febrile neutropenia" (11 (14%)) plus the concurrent events of "fever" and "neutropenia" (16 (21%)). In the event of febrile neutropenia, evaluate for infection and manage with broad spectrum antibiotics, fluids, and other supportive care as medically indicated.
In immunosuppressed patients, life-threatening and fatal opportunistic infections have been reported. The possibility of rare infectious etiologies (e.g., fungal and viral infections such as HHV-6 and progressive multifocal leukoencephalopathy) should be considered in patients with neurologic events and appropriate diagnostic evaluations should be performed.
Hepatitis B Reactivation
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients treated with drugs directed against B cells. Perform screening for HBV, hepatitis C virus (HCV), and human immunodeficiency virus (HIV) in accordance with clinical guidelines before collection of cells for manufacturing.
5.6 Prolonged Cytopenias
Patients may exhibit cytopenias for several weeks following lymphodepleting chemotherapy and TECARTUS infusion. In patients with MCL, Grade 3 or higher cytopenias not resolved by Day 30 following TECARTUS infusion occurred in 55% (45/82) of patients and included thrombocytopenia (38%), neutropenia (37%), and anemia (17%). In patients with ALL who were responders to TECARTUS treatment, Grade 3 or higher cytopenias not resolved by Day 30 following TECARTUS infusion occurred in 20% (7/35) of the patients and included neutropenia (12%) and thrombocytopenia (12%); Grade 3 or higher cytopenias not resolved by Day 60 following TECARTUS infusion occurred in 11% (4/35) of the patients and included neutropenia (9%) and thrombocytopenia (6%). Monitor blood counts after TECARTUS infusion.
5.7 Hypogammaglobulinemia
B cell aplasia and hypogammaglobulinemia can occur in patients receiving treatment with TECARTUS. Hypogammaglobulinemia was reported in 16% (13/82) of patients with MCL and 9% (7/78) of patients with ALL. Monitor immunoglobulin levels after treatment with TECARTUS and manage using infection precautions, antibiotic prophylaxis, and immunoglobulin replacement.
The safety of immunization with live viral vaccines during or following TECARTUS treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least six weeks prior to the start of lymphodepleting chemotherapy, during TECARTUS treatment, and until immune recovery following treatment with TECARTUS.
5.8 Secondary Malignancies
Patients treated with TECARTUS may develop secondary malignancies. T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies. Mature T cell malignancies, including CAR-positive tumors, may present as soon as weeks following infusion, and may include fatal outcomes. [see Boxed Warning, Adverse Reactions (6.3), Patient Counseling Information (17)].
Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Kite at 1-844-454-KITE (5483) to obtain instructions on patient samples to collect for testing.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Cytokine Release Syndrome [see Warnings and Precautions (5.1)]
- Neurologic Toxicities [see Warnings and Precautions (5.2)]
- Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome [see Warnings and Precautions (5.3)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
- Severe Infections [see Warnings and Precautions (5.5)]
- Prolonged Cytopenias [see Warnings and Precautions (5.6)]
- Hypogammaglobulinemia [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Patients with Relapsed/Refractory Mantle Cell Lymphoma (MCL)
The safety of TECARTUS was evaluated in a Phase 2 single-arm clinical study (ZUMA-2) in which a total of 82 patients with relapsed/refractory MCL received a single dose of CAR-positive viable T cells (2 × 106 or 0.5 × 106 anti-CD19 CAR T cells/kg) that was weight-based [see Clinical Studies (14.1)].
The most common adverse reactions (incidence ≥ 20%) were fever, CRS, hypotension, encephalopathy, fatigue, tachycardia, arrhythmia, infection with pathogen unspecified, chills, hypoxia, cough, tremor, musculoskeletal pain, headache, nausea, edema, motor dysfunction, constipation, diarrhea, decreased appetite, dyspnea, rash, insomnia, pleural effusion, and aphasia. Serious adverse reactions occurred in 66% of patients. The most common serious adverse reactions (> 2%) were encephalopathy, fever, infection with pathogen unspecified, CRS, hypoxia, aphasia, renal insufficiency, pleural effusion, respiratory failure, bacterial infections, dyspnea, fatigue, arrhythmia, tachycardia, and viral infections.
The most common (≥ 10%) Grade 3 or higher reactions were anemia, neutropenia, thrombocytopenia, hypotension, hypophosphatemia, encephalopathy, leukopenia, hypoxia, fever, hyponatremia, hypertension, infection with pathogen unspecified, pneumonia, hypocalcemia, and lymphopenia.
Table 3 summarizes the adverse reactions that occurred in at least 10% of patients treated with TECARTUS and Table 4 describes the laboratory abnormalities of Grade 3 or 4 that occurred in at least 10% of patients.
Table 3. Summary of Adverse Reactions Observed in at Least 10% of Patients Treated with TECARTUS in ZUMA-2 (N=82) Adverse Reaction Any Grade (%) Grade 3 or Higher (%) - *
- Coagulopathy includes coagulopathy, disseminated intravascular coagulation, international normalized ratio increased.
- †
- Tachycardias includes tachycardia, sinus tachycardia.
- ‡
- Bradycardias includes bradycardia, sinus bradycardia.
- §
- Non-ventricular arrhythmias includes atrial fibrillation, atrial flutter, cardiac flutter, palpitations, supraventricular tachycardia.
- ¶
- Abdominal pain includes abdominal pain, abdominal pain lower, abdominal pain upper, abdominal tenderness.
- #
- Oral pain includes oral pain, gingival pain, lip pain, oral mucosal erythema, oropharyngeal pain.
- Þ
- Vomiting includes vomiting, retching.
- ß
- Fatigue includes fatigue, lethargy, malaise.
- à
- Edema includes eyelid edema, face edema, generalized edema, localised edema, edema, edema peripheral, periorbital edema, peripheral swelling, scrotal edema, swelling face.
- è
- Pain includes pain, allodynia, dysaesthesia, ear pain, facial pain, non-cardiac chest pain.
- ð
- Hypogammaglobulinemia includes hypogammaglobulinemia, blood immunoglobulin G decreased.
- ø
- Musculoskeletal pain includes musculoskeletal pain, arthralgia, back pain, bone pain, dysarthria, flank pain, groin pain, myalgia, neck pain, pain in extremity.
- ý
- Motor dysfunction includes asthenia, intensive care acquired weakness, mobility decreased, muscle twitching, muscular weakness, myopathy.
- £
- Encephalopathy includes encephalopathy, altered state of consciousness, amnesia, balance disorder, cognitive disorder, confusional state, disturbance in attention, dysgraphia, dyskinesia, memory impairment, mental status changes, neurotoxicity, somnolence.
- ¥
- Headache includes headache, migraine.
- Œ
- Aphasia includes aphasia, communication disorder.
- œ
- Dizziness includes dizziness, presyncope, syncope.
- Ɖ
- Neuropathy includes hyperaesthesia, neuropathy peripheral, paraesthesia, paraesthesia oral.
- A
- Delirium includes delirium, agitation, disorientation, hallucination, hypomania, irritability, nervousness, personality change.
- B
- Renal insufficiency includes acute kidney injury, blood creatinine increased.
- C
- Urine output decreased includes urine output decreased, urinary retention.
- D
- Cough includes cough, upper-airway cough syndrome.
- E
- Dyspnea includes dyspnea, dyspnea exertional.
- F
- Rash includes rash, erythema, rash erythematous, rash maculo-papular, rash pustular.
- G
- Hypotension includes hypotension, orthostatic hypotension.
- H
- Thrombosis includes thrombosis, deep vein thrombosis, embolism, pulmonary embolism.
Blood and Lymphatic System Disorders Coagulopathy * 10 2 Cardiac Disorders Tachycardias † 45 0 Bradycardias ‡ 10 0 Non-ventricular Arrhythmias § 10 4 Gastrointestinal Disorders Nausea 35 1 Constipation 29 0 Diarrhea 28 5 Abdominal pain ¶ 17 0 Oral pain # 16 0 Vomiting Þ 13 0 Dysphagia 10 2 General Disorders and Administration Site Conditions Fever 94 15 Fatigue ß 48 1 Chills 41 0 Edema à 35 2 Pain è 17 2 Immune System Disorders Cytokine release syndrome 91 18 Hypogammaglobulinemia ð 16 1 Infections and Infestations Infection with pathogen unspecified 43 24 Viral infections 18 4 Bacterial infections 13 6 Metabolism and Nutrition Disorders Decreased appetite 26 0 Musculoskeletal and Connective Tissue Disorders Musculoskeletal pain ø 37 2 Motor dysfunction ý 17 4 Nervous System Disorders Encephalopathy £ 51 24 Tremor 38 2 Headache ¥ 35 1 Aphasia Œ 20 7 Dizziness œ 18 6 Neuropathy Ɖ 13 2 Psychiatric Disorders Insomnia 21 0 Delirium A 18 5 Anxiety 16 0 Renal and Urinary Disorders Renal insufficiency B 18 9 Urine output decreased C 11 1 Respiratory, Thoracic and Mediastinal Disorders Hypoxia 40 20 Cough D 38 0 Dyspnea E 24 6 Pleural effusion 21 5 Skin and Subcutaneous Tissue Disorders Rash F 22 4 Vascular Disorders Hypotension G 57 27 Hypertension 18 11 Thrombosis H 17 4 Other clinically important adverse reactions that occurred in less than 10% of patients treated with TECARTUS include the following:
- Gastrointestinal disorders: dry mouth (7%)
- Infections and infestations disorders: fungal infections (9%)
- Metabolism and nutrition disorders: dehydration (6%)
- Nervous system disorders: ataxia (7%), seizure (5%), increased intracranial pressure (2%)
- Respiratory, thoracic and mediastinal disorders: respiratory failure (6%), pulmonary edema (4%)
- Skin and subcutaneous tissue disorders: rash (9%)
- Vascular disorders: hemorrhage (7%)
Table 4. Grade 3 or 4 Laboratory Abnormalities Occurring in ≥ 10% of Patients in ZUMA-2 Following TECARTUS Infusion (N = 82) Grades 3 or 4 (%) Leukopenia 95 Neutropenia 95 Lymphopenia 86 Thrombocytopenia 63 Anemia 55 Hypophosphatemia 30 Hypocalcemia 21 Blood uric acid increased 17 Hyponatremia 16 Aspartate Aminotransferase increased 15 Alanine Aminotransferase increased 15 Hypokalemia 10 Patients with Relapsed/Refractory B-cell precursor Acute Lymphoblastic Leukemia (ALL)
The safety of TECARTUS was evaluated in a Phase 1/2 open-label, multicenter study (ZUMA-3) in which a total of 78 patients with relapsed/refractory ALL received a single dose of CAR-positive T cells (1 × 106 anti-CD19 CAR T cells/kg) that was weight-based [see Clinical Studies (14.2)].
The most common non-laboratory adverse reactions (≥ 20%) were fever, cytokine release syndrome, hypotension, encephalopathy, tachycardia, nausea, chills, headache, fatigue, febrile neutropenia, diarrhea, musculoskeletal pain, hypoxia, rash, edema, tremor, infection with pathogen unspecified, constipation, decreased appetite, and vomiting. The most common serious adverse reactions (≥ 2%) were cytokine release syndrome, febrile neutropenia, hypotension, encephalopathy, fever, infection with pathogen unspecified, hypoxia, tachycardia, bacterial infections, respiratory failure, seizure, diarrhea, dyspnea, fungal infections, viral infections, coagulopathy, delirium, fatigue, hemophagocytic lymphohistiocytosis, musculoskeletal pain, edema, and paraparesis.
The most common (≥ 10%) Grade 3 or 4 reactions were fever, febrile neutropenia, hypotension, encephalopathy, cytokine release syndrome, hypoxia, and infection with pathogen unspecified. Fatal adverse reactions occurred in 5% (4/78) of patients including cerebral edema, sepsis, and fungal pneumonia. Of the 4 patients who had fatal adverse reactions: one patient with fatal pneumonia had pre-existing pneumonia prior to study enrollment, and one patient with fatal sepsis had prolonged cytopenia and immunosuppression from prior therapies and underlying disease.
Table 5 summarizes the adverse reactions (excluding laboratory abnormalities) that occurred in at least 10% of patients treated with TECARTUS and Table 6 describes the laboratory abnormalities of Grade 3 or 4 that occurred in at least 10% of patients.
Table 5. Summary of Adverse Reactions Observed in at Least 10% of Patients Treated with TECARTUS in ZUMA-3 (N=78) Adverse Reaction Any Grade (%) Grade 3 or Higher (%) - *
- Febrile neutropenia includes febrile neutropenia (11 (14%)) and fever occurring concurrently with neutropenia events (16 (21%)).
- †
- Coagulopathy includes blood fibrinogen decreased, coagulopathy, disseminated intravascular coagulation, hypofibrinogenemia, international normalized ratio increased.
- ‡
- Tachycardias includes sinus tachycardia, tachycardia.
- §
- Arrhythmia includes arrhythmia, arrhythmia supraventricular, atrial fibrillation, atrial flutter, atrial tachycardia, bradycardia, cardiac arrest, electrocardiogram QT prolonged, electrocardiogram T wave inversion, pulseless electrical activity, sinus bradycardia, supraventricular tachycardia, ventricular tachycardia.
- ¶
- Abdominal pain includes abdominal discomfort, abdominal pain, abdominal pain upper.
- #
- Edema includes face edema, fluid overload, generalized edema, edema, edema peripheral, swelling face, tongue edema.
- Þ
- Fatigue includes asthenia, cancer fatigue, fatigue, malaise.
- ß
- Infection with pathogen unspecified includes, catheter bacteremia, conjunctivitis, device related infection, mucosal infection, nasopharyngitis, parotitis, pneumonia, sepsis, septic shock, sinusitis, upper respiratory tract infection, urinary tract infection.
- à
- Bacterial infections includes bacteremia, bacterial disease carrier, cellulitis, cellulitis of male external genital organ, clostridial infection, clostridium difficile colitis, clostridium difficile infection, enterococcal bacteremia, enterococcal infection, escherichia bacteremia, escherichia infection, escherichia sepsis, pseudomonas infection, staphylococcal bacteremia, staphylococcal infection, wound infection staphylococcal.
- è
- Fungal infections includes bronchopulmonary aspergillosis, candida infection, fungal skin infection, mycotic oral candidiasis, osteomyelitis fungal, pneumocystis jirovecii pneumonia, pneumonia fungal, sinusitis fungal.
- ð
- Musculoskeletal pain includes arthralgia, back pain, bone pain, coccydynia, muscle strain, musculoskeletal chest pain, musculoskeletal pain, myalgia, neck pain, non-cardiac chest pain, pain in extremity.
- ø
- Encephalopathy includes altered state of consciousness, aphasia, cognitive disorder, confusional state, depressed level of consciousness, disturbance in attention, dysarthria, dysgraphia, encephalopathy, immune effector cell-associated neurotoxicity syndrome, memory impairment, mental status changes, slow response to stimuli, slow speech, somnolence, speech disorder.
- ý
- Dizziness includes dizziness, syncope.
- £
- Delirium includes agitation, delirium, delusion, disorientation, hallucination.
- ¥
- Cough includes cough, productive cough.
- Œ
- Rash includes catheter site urticaria, dermatitis bullous, drug eruption, pruritus, rash, rash macular, rash maculo-papular, rash pustular, toxic skin eruption, urticaria.
- œ
- Hypotension includes hypotension, orthostatic hypotension.
- Ɖ
- Hemorrhage includes conjunctival hemorrhage, contusion, epistaxis, gastric hemorrhage, hematoma, hematoma muscle, hemorrhage intracranial, hemorrhoidal hemorrhage, menorrhagia, petechiae, pulmonary alveolar hemorrhage, retinal hemorrhage, vaginal hemorrhage, vitreous hemorrhage.
Blood and Lymphatic System Disorders Febrile Neutropenia* 35 35 Coagulopathy† 17 5 Cardiac Disorders Tachycardias‡ 63 6 Arrhythmia§ 15 1 Gastrointestinal Disorders Nausea 41 1 Diarrhea 32 6 Abdominal pain¶ 19 0 Constipation 24 0 Vomiting 21 3 General Disorders and Administration Site Conditions Fever 96 38 Chills 40 0 Edema# 29 5 FatigueÞ 37 1 Pain 13 1 Immune System Disorders Cytokine release syndrome 92 26 Infections and Infestations Infection with pathogen unspecifiedß 28 22 Bacterial infectionsà 15 8 Fungal infectionsè 13 5 Metabolism and Nutrition Disorders Decreased appetite 22 1 Musculoskeletal and Connective Tissue Disorders Musculoskeletal painð 32 5 Muscular weakness 14 1 Nervous System Disorders Encephalopathyø 63 27 Headache 38 1 Tremor 29 1 Dizzinessý 13 1 Psychiatric Disorders Delirium£ 18 5 Anxiety 12 0 Insomnia 13 0 Respiratory, Thoracic and Mediastinal Disorders Hypoxia 31 23 Cough¥ 12 0 Dyspnea 12 1 Skin and Subcutaneous Tissue Disorders RashŒ 31 0 Vascular Disorders Hypotensionœ 69 33 HemorrhageƉ 13 4 Hypertension 13 6 Other clinically important adverse reactions that occurred in less than 10% of patients treated with TECARTUS include the following:
- Cardiac disorder: cardiac failure (4%), palpitations (3%)
- Eye disorders: visual impairment (9%)
- Gastrointestinal disorders: dry mouth (6%), dysphagia (4%), oral pain (1%)
- Immune system disorders: hypogammaglobulinemia (9%), hemophagocytic lymphohistiocytosis (4%), drug hypersensitivity (1%)
- Infections and infestations: viral infections (6%)
- Metabolism and nutrition disorders: dehydration (5%), tumor lysis syndrome (1%)
- Musculoskeletal and connective tissue disorders: muscle spasms (4%), musculoskeletal stiffness (3%)
- Nervous system disorders: seizure (8%), ataxia (5%), peripheral neuropathy (4%), myoclonus (3%), paraparesis (3%), brain edema (1%), brain herniation (1%), cauda equina syndrome (1%), monoplegia (1%)
- Renal and urinary disorders: renal impairment (6%)
- Respiratory, thoracic and mediastinal disorders: respiratory failure (9%), pulmonary edema (6%), pleural effusion (4%), pneumonitis (4%)
- Skin and subcutaneous tissue disorders: skin lesion (4%), decubitus ulcer (3%), dry skin (3%), skin ulcer (3%), alopecia (1%), hyperhidrosis (1%), skin hyperpigmentation (1%)
- Vascular disorders: thrombosis (4%)
Table 6. Grade 3 or 4 Laboratory Abnormalities Occurring in ≥ 10% of Patients in ZUMA-3 Following TECARTUS Infusion (N = 78) Grades 3 or 4 (%) Leukopenia 99 Neutropenia 97 Lymphopenia 96 Thrombocytopenia 87 Anemia 77 Hypophosphatemia 47 Alanine aminotransferase increased 31 Aspartate aminotransferase increased 23 Hyperglycemia 22 Hypocalcemia 22 Blood uric acid increased 19 Direct bilirubin increased 19 Hyponatremia 19 Hypokalemia 13 Hyperbilirubinemia 10 6.2 Immunogenicity
TECARTUS has the potential to induce anti-product antibodies, which has been evaluated using an enzyme-linked immunosorbent assay (ELISA) for the detection of binding antibodies against FMC63, the originating antibody of the anti-CD19 CAR. To date, no anti-CAR T cell antibody immunogenicity has been observed in ZUMA-2. Based on an initial screening assay in ZUMA-2, 17 of 82 patients tested positive for antibodies at any timepoint; however, a confirmatory orthogonal cell-based assay demonstrated that all 17 patients were antibody negative at all timepoints tested. Based on an initial screening assay in ZUMA-3, 16 of 100 patients tested positive for antibodies at any timepoint. Among patients with evaluable samples for confirmatory testing, two patients were confirmed to be antibody-positive after treatment. One of the two patients had a confirmed positive antibody result at Month 6. The second patient had a confirmed antibody result at retreatment Day 28 and Month 3. There is no evidence that the kinetics of initial expansion and persistence of TECARTUS, or the safety or effectiveness of TECARTUS, were altered in these patients.
6.3 Postmarketing Experience
Because adverse events to marketed products are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to product exposure.
The following adverse event has been identified during postmarketing use of TECARTUS.
Immune System Disorders: Infusion related reaction
The following adverse event has been identified during postmarketing use of BCMA- or CD19-directed genetically modified autologous T cell immunotherapies:
Neoplasms: T cell malignancies
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data with TECARTUS use in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with TECARTUS to assess whether TECARTUS can cause fetal harm when administered to a pregnant woman. It is not known if TECARTUS has the potential to be transferred to the fetus. Based on the mechanism of action of TECARTUS, if the transduced cells cross the placenta, they may cause fetal toxicity, including B cell lymphocytopenia. Therefore, TECARTUS is not recommended for women who are pregnant. Pregnancy after TECARTUS infusion should be discussed with the treating physician.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% – 4% and 15% – 20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of TECARTUS in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for TECARTUS and any potential adverse effects on the breastfed infant from TECARTUS or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy status of females with reproductive potential should be verified. Sexually active females of reproductive potential should have a negative pregnancy test prior to starting treatment with TECARTUS.
Contraception
See the Prescribing Information for fludarabine and cyclophosphamide for information on the need for effective contraception in patients who receive the lymphodepleting chemotherapy.
There are insufficient exposure data to provide a recommendation concerning duration of contraception following treatment with TECARTUS.
8.4 Pediatric Use
The safety and efficacy of TECARTUS have not been established in pediatric patients.
8.5 Geriatric Use
Of the 82 patients treated with TECARTUS for MCL, 42 (51%) were 65 years of age and over. Of the 78 patients treated with TECARTUS for ALL, 12 (15%) were 65 years of age and over. No overall differences in safety or effectiveness were observed between these patients and younger patients.
-
11 DESCRIPTION
TECARTUS (brexucabtagene autoleucel) is a CD19-directed genetically modified autologous T cell immunotherapy. To prepare TECARTUS, a patient's own T cells are harvested and genetically modified ex vivo by retroviral transduction to express a chimeric antigen receptor (CAR) comprising a murine anti-CD19 single-chain variable fragment (scFv) linked to CD28 and CD3-zeta co-stimulatory domains. The anti-CD19 CAR T cells are expanded and infused back into the patient, where they can recognize and eliminate CD19-expressing target cells.
TECARTUS is prepared from the patient's peripheral blood mononuclear cells, which are obtained via a standard leukapheresis procedure. The mononuclear cells are enriched for T cells and activated with anti-CD3 and anti-CD28 antibodies in the presence of IL-2, then transduced with a replication-incompetent retroviral vector containing the anti-CD19 CAR transgene. The transduced T cells are expanded in cell culture, washed, formulated into a suspension, and cryopreserved. The manufacture of TECARTUS includes a T cell enrichment step that may reduce the likelihood of circulating CD19-expressing tumor cells in patients' leukapheresis material driving the activation, expansion, and exhaustion of the anti-CD19 CAR T cells during the ex vivo manufacturing process. The product must pass a sterility test before release for shipping as a frozen suspension in a patient-specific infusion bag. The product is thawed prior to infusion [see Dosage and Administration (2.2), How Supplied/Storage and Handling (16)].
In addition to T cells, TECARTUS may contain natural killer (NK) cells. The formulation contains CryoStor (dimethyl sulfoxide [DMSO], final concentration, 5%), sodium chloride (NaCl), and Human Serum Albumin (HSA).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
TECARTUS, a CD19-directed genetically modified autologous T cell immunotherapy, binds to CD19-expressing cancer cells and normal B cells. Studies demonstrated that following anti-CD19 CAR T cell engagement with CD19-expressing target cells, the CD28 and CD3-zeta co-stimulatory domains activate downstream signaling cascades that lead to T cell activation, proliferation, acquisition of effector functions, and secretion of inflammatory cytokines and chemokines. This sequence of events leads to killing of CD19-expressing cells.
12.2 Pharmacodynamics
After TECARTUS infusion, pharmacodynamic responses were evaluated over a four-week interval by measuring transient elevation of cytokines, chemokines, and other molecules in blood. Levels of cytokines and chemokines such as IL-6, IL-8, IL-10, IL-15, TNF-α, IFN-γ, and sIL2Rα were analyzed. Peak elevation was generally observed within 8 days after infusion, and levels generally returned to baseline within 28 days.
Due to the on-target effect of TECARTUS, a period of B cell aplasia is expected.
12.3 Pharmacokinetics
Following infusion (target dose of 2 × 106 anti-CD19 CAR T cells/kg) of TECARTUS in ZUMA-2, anti-CD19 CAR T cells exhibited an initial rapid expansion followed by a decline to near baseline levels by three months. Peak levels of anti-CD19 CAR T cells occurred within the first 15 days after TECARTUS infusion. Following infusion (target dose of 1 × 106 anti-CD19 CAR T cells/kg) of TECARTUS in ZUMA-3 (Phase 2), anti-CD19 CAR T cells exhibited an initial rapid expansion followed by a decline to near baseline levels by 6 months. Median anti- CD19 CAR T cell time to peak was 15 days after TECARTUS infusion.
Description of Pharmacokinetics in Adult r/r MCL
The number of anti-CD19 CAR T cells in blood was associated with objective response [complete remission (CR) or partial remission (PR)]. Median peak anti-CD19 CAR T cell level in responders was 102.4 cells/µL (range: 0.2 to 2589.5 cells/µL; n = 51), and in nonresponders was 12.0 cells/µL (range: 0.2 to 1364.0 cells/µL, n = 8). The median AUCDay 0–28 in patients with an objective response was 1487.0 cells/µL∙days (range: 3.8 to 2.77E+04 cells/µL∙days; n = 51) versus 169.5 cells/µL∙days in nonresponders (range: 1.8 to 1.17E+04 cells/µL∙days; n = 8).
Median peak anti-CD19 CAR T cell and AUC0–28 levels in patients who received neither corticosteroids nor tocilizumab (peak: 24.7 cells/µL; AUC0–28: 360.4 cells/µL∙days, n = 18) was similar to patients who received corticosteroids alone (peak: 24.2 cells/µL; AUC0–28: 367.8 cells/µL∙days, n = 2); both groups were lower than patients who received tocilizumab alone (peak: 86.5 cells/µL; AUC0–28: 1188.9 cells/µL∙days, n = 10); the highest exposure was in patients who received both corticosteroids and tocilizumab (peak: 167.2 cells/µL; AUC0–28: 1996.0 cells/µL∙days, n = 37).
Median peak anti-CD19 CAR T cell values were 74.1 cells/µL in patients ≥ 65 years of age (n = 39) and 112.5 cells/µL in patients < 65 years of age (n = 28). Median anti-CD19 CAR T cell AUCDay 0–28 values were 876.5 cells/µL∙day in patients ≥ 65 years of age and 1640.2 cells/µL∙day in patients < 65 years of age.
Gender had no significant impact on AUC Day 0–28 and Cmax of TECARTUS.
Description of Pharmacokinetics in Adult r/r B-cell precursor ALL
Median peak anti-CD19 CAR T cell levels over time by best overall response per independent review was 38.4 cells/µL (range: 1.31 to 1533.4 cells/µL; n = 32) in patients who had overall complete remission (CR+CRi), and 0.5 cells/µL (range: 0.00 to 183.5 cells/µL, n = 17) in patients who had non-complete remission. The median AUC0–28 in patients who had overall complete remission (CR+CRi) was 424.0 cells/µL∙days (range: 14.12 to 19,390.4 cells/µL∙days; n = 32) vs 7.9 cells/µL∙days in patients who had non-complete remission (range: 0.00 to 889.0 cells/µL∙days; n=17).
Median peak anti-CD19 CAR T cell and AUC0–28 levels in patients who received neither corticosteroids nor tocilizumab (peak 5.7 cells/µL; AUC0–28: 60.7 cells/µL∙days, n=11) were higher than patients who received corticosteroids alone (peak: 36.2 cells/ µL; AUC0–28: 423.1 cells/µL∙days, n= 1); both groups were lower than evaluable patients who received tocilizumab alone (peak 11.2 cells/ µL; AUC0–28: 137.4 cells/µL∙days, n=9); the highest exposure was in evaluable patients who received both corticosteroids and tocilizumab (peak: 49.2 cells/µL; AUC0–28: 454.1 cells/ µL∙days, n=34).
Hepatic and renal impairment studies of TECARTUS were not conducted.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Relapsed or Refractory Mantle Cell Lymphoma
A single-arm, open-label, multicenter trial (ZUMA-2; NCT02601313) evaluated the efficacy and safety of a single infusion of TECARTUS in adult patients with relapsed or refractory mantle cell lymphoma (MCL) who had previously received anthracycline- or bendamustine-containing chemotherapy, an anti-CD20 antibody, and a Bruton tyrosine kinase inhibitor (BTKi; ibrutinib or acalabrutinib). Eligible patients also had disease progression after their last regimen or refractory disease to their most recent therapy. The study excluded patients with active or serious infections, prior allogeneic hematopoietic stem cell transplant (HSCT), detectable cerebrospinal fluid malignant cells or brain metastases, and any history of central nervous system (CNS) lymphoma or CNS disorders.
Seventy-four patients were leukapheresed, five (7%) of whom did not begin conditioning chemotherapy or receive TECARTUS: three (4%) experienced manufacturing failure, one (1%) died of progressive disease, and one (1%) withdrew from the study. One patient (1%) received lymphodepleting chemotherapy but did not receive TECARTUS due to ongoing active atrial fibrillation. Sixty-eight of the patients who were leukapheresed received a single infusion of TECARTUS, and 60 of these patients were followed for at least six months after their first objective disease response, qualifying them as efficacy-evaluable. Among the 60 efficacy-evaluable patients, 2 × 106 CAR-positive viable T cells/kg were administered to 54 (90%). The remaining six (10%) patients received doses of 1.0, 1.6, 1.8, 1.8, 1.9, and 1.9 × 106 CAR-positive viable T cells/kg.
Of the 60 efficacy-evaluable patients, the median age was 65 years (range: 38 to 79 years), 51 (85%) were male, and 56 (93%) were white. Most (50 patients; 83%) had stage IV disease. Twenty patients (33% of 60) had baseline bone marrow examinations performed per protocol; of these, ten (50%) were negative, eight (40%) were positive, and two (10%) were indeterminate. The median number of prior therapies among all 60 efficacy-evaluable patients was three (range: two to five). Twenty-six (43%) of the patients had relapsed after or were refractory to autologous HSCT. Twenty-one (35%) had relapsed after their last therapy for MCL, while 36 (60%) were refractory to their last therapy for MCL. Among the 60 efficacy-evaluable patients, 14 (23%) had blastoid MCL. Following leukapheresis and prior to administration of TECARTUS, 21 (35%) of the 60 patients received bridging therapy. Sixteen (27%) were treated with a BTKi, 9 (15%) with a corticosteroid, and 4 (7%) with both a BTKi and a corticosteroid.
Among the 60 efficacy-evaluable patients, the median time from leukapheresis to product delivery was 15 days (range: 11 to 28 days), and the median time from leukapheresis to product infusion was 27 days (range: 19 to 63 days). The protocol-defined lymphodepleting chemotherapy regimen of cyclophosphamide 500 mg/m2 intravenously and fludarabine 30 mg/m2 intravenously, both given on each of the fifth, fourth, and third days before TECARTUS infusion, was administered to 53 (88%) of the 60 efficacy-evaluable patients. The remaining seven patients (12%) either received lymphodepletion over four or more days or received TECARTUS four or more days after completing lymphodepletion. All treated patients received TECARTUS infusion on Day 0 and were hospitalized until at least Day 7.
The primary endpoint of objective response rate (ORR) per the Lugano Classification (2014) in patients treated with TECARTUS as determined by an independent review committee is provided in Table 7. The median time to response was 28 days (range: 24 to 92 days) with a median follow-up time for DOR of 8.6 months.
Table 7. Efficacy Results in Adult Patients with Relapsed/Refractory MCL Efficacy-Evaluable Patients
N = 60All Leukapheresed Patients (ITT)
N = 74CI, confidence interval; CR, complete remission; DOR, duration of response; NE, not estimable; NR, not reached; PR, partial remission. Response Rate Objective Response Rate*, n (%) [95% CI] 52 (87%) [75, 94] 59 (80%) [69, 88] Complete Remission Rate, n (%) [95% CI] 37 (62%) [48, 74] 41 (55%) [43, 67] Partial Remission Rate, n (%) [95% CI] 15 (25%) [15, 38] 18 (24%) [15, 36] Duration of Response (DOR) Median in months [95% CI]
Range† in monthsNR [8.6, NE]
0.0+, 29.2+NR [8.6, NE]
0.0+, 29.2+DOR, if best response is CR, median in months [95% CI]
Range† in monthsNR [13.6, NE]
1.9+, 29.2+NR [13.6, NE]
0.0+, 29.2+DOR, if best response is PR, median in months [95% CI]
Range† in months2.2 [1.5, 5.1]
0.0+, 22.1+2.2 [1.5, 5.1]
0.0+, 22.1+Median Follow-up for DOR in months‡ 8.6 8.1 14.2 Relapsed or Refractory B-cell precursor Acute Lymphoblastic Leukemia
The efficacy of TECARTUS was evaluated in ZUMA-3 (NCT02614066), an open-label, single-arm, multicenter trial in adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL). Eligible patients were adults with primary refractory ALL, first relapse following a remission lasting ≤ 12 months, relapsed or refractory ALL after second-line or higher therapy, or relapsed or refractory ALL at least 100 days after allogeneic stem cell transplantation (HSCT). The study excluded patients with active or serious infections, active graft-vs-host disease or taking immunosuppressive medications within 4 weeks prior to enrollment, and any history of CNS disorders, including CNS-2 disease with neurologic changes and CNS-3 disease irrespective of neurological changes. Treatment consisted of lymphodepleting chemotherapy (fludarabine 25 mg/m2 iv daily on Days -4, -3 and -2; cyclophosphamide 900 mg/m2 iv on Day -2) followed by a single intravenous infusion of TECARTUS at a target dose of 1 × 106 anti-CD19 CAR T cells/kg (maximum 1 × 108 cells) on Day 0. All treated patients were hospitalized until at least Day 7.
Seventy-one patients were enrolled and leukapheresed; six of these patients did not receive TECARTUS due to manufacturing failure, eight patients were not treated primarily due to adverse events following leukapheresis, two patients underwent leukapheresis and received lymphodepleting chemotherapy but were not treated with TECARTUS, and one patient treated with TECARTUS was inevaluable for efficacy. Among the remaining 54 efficacy-evaluable patients, the median time from leukapheresis to product delivery was 16 days (range: 11 to 39 days) and the median time from leukapheresis to TECARTUS infusion was 29 days (range: 20 to 60 days).
Of the 54 patients who were efficacy-evaluable, the median age was 40 years (range: 19 to 84 years), 61% were male, and 67% were White, 6% were Asian, 2% were Black or African American, and 2% were American Indian or Alaska Native. At enrollment, 46% had refractory relapse, 26% had primary refractory disease, 20% had untreated second or later relapse, and 7% had first untreated relapse. Among prior therapies, 43% of patients were previously treated with allo-SCT, 46% with blinatumomab, and 22% with inotuzumab. Twenty-six percent of patients were Philadelphia chromosome positive (Ph+). Fifty (93%) patients had received bridging therapy between leukapheresis and lymphodepleting chemotherapy to control disease burden.
The efficacy of TECARTUS was established on the basis of complete remission (CR) within 3 months after infusion and the duration of CR (DOCR). Twenty-eight (51.9%) of the 54 evaluable patients achieved CR, and with a median follow-up for responders of 7.1 months, the median DOCR was not reached (Table 8). The median time to CR was 56 days (range: 25 to 86 days). All efficacy-evaluable patients had potential follow-up for ≥ 10 months with a median actual follow-up time of 12.3 months (range: 0.3 to 22.1 months).
Table 8. Efficacy Results in Adult Patients with Relapsed/Refractory B-cell precursor ALL Efficacy Evaluable Patients*
N= 54All Leukapheresed Patients
N = 71CI, confidence interval; CR, complete remission; CRi, complete remission with incomplete blood count recovery; DOR, duration of remission; NE, not estimable; NR, not reached, OCR, overall complete remission; NE, not estimable OCR rate (CR + CRi), n (%) [95% CI] 35 (64.8) [51, 77] 36 (50.7) [39, 63] CR rate, n (%) [95% CI] 28 (51.9) [37.8, 65.7] 29 (40.9) [29.3, 53.2] Duration of Remission, Median in months [95% CI]
(Range† in months)13.6 [9.4, NE]
(0.03+, 16.07+)13.6 [8.7, NE]
(0.03+, 16.07+)DOR, if best response is CR, median in months [95% CI]
(Range in months)NR [9.6, NE]
(0.03+, 16.07+)13.6 [9.4, NE]
(0.03+, 16.07+)DOR, if best response is CRi, median in months [95% CI]
(Range in months)8.7 [1.0, NE]
(0.03+, 10.15+)8.7 [1.0, NE]
(0.03+, 10.15+)Median Follow-up for CR in months 7.1 (0.03+, 16.1+) 5.0 (0.03+, 16.1+) - 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
TECARTUS is supplied in an infusion bag containing approximately 68 mL of frozen suspension of genetically modified autologous T cells in 5% DMSO and human serum albumin.
Each TECARTUS infusion bag is individually packed in a metal cassette. TECARTUS is supplied in a liquid nitrogen dry shipper.
Indication Infusion Bag NDC number Metal Cassette NDC number MCL 71287-219-01 71287-219-02 ALL 71287-220-01 71287-220-02 - Match the identity of the patient with the patient identifiers on the cassette and infusion bag upon receipt.
- Store TECARTUS frozen in the vapor phase of liquid nitrogen (less than or equal to -150°C). TECARTUS may be stored for a single time at -80°C (+/- 10°C), for up to 90 days, and not exceeding the labeled expiration date. Do not return TECARTUS to storage in the vapor phase of liquid nitrogen after storage at -80°C.
- Thaw before using [see Dosage and Administration (2)].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Ensure that patients understand the risk of manufacturing failure (4% in clinical trial). In case of a manufacturing failure, a second manufacturing of TECARTUS may be attempted. In addition, while the patient awaits the product, additional chemotherapy (not the lymphodepletion) may be necessary and may increase the risk of adverse events during the pre-infusion period.
Advise patients to seek immediate attention for any of the following:
- Cytokine Release Syndrome (CRS) - Signs or symptoms associated with CRS, including fever, chills, fatigue, tachycardia, nausea, hypoxia, and hypotension [see Warnings and Precautions (5.1) and Adverse Reactions (6)].
- Neurologic Toxicities - Signs or symptoms associated with neurologic events, including encephalopathy, seizures, changes in level of consciousness, speech disorders, tremors, and confusion [see Warnings and Precautions (5.2) and Adverse Reactions (6)].
- Severe Infections - Signs or symptoms associated with infection [see Warnings and Precautions (5.5) and Adverse Reactions (6)].
- Prolonged Cytopenias - Signs or symptoms associated with bone marrow suppression, including neutropenia, anemia, thrombocytopenia, or febrile neutropenia [see Warnings and Precautions (5.6) and Adverse Reactions (6)].
- Secondary Malignancies - Secondary malignancies, including T cell malignancies, have occurred following treatment with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies [see Boxed Warning, Warnings and Precautions (5.8), Adverse Reactions (6.3)].
Advise patients of the need to:
- Avoid driving for at least 2 weeks.
- Contact Kite at 1-844-454-KITE (5483) if they are diagnosed with a secondary malignancy [see Warnings and Precautions (5.8)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: June 2025 MEDICATION GUIDE
TECARTUS (pronounced tek-ahr-tuhs)
(brexucabtagene autoleucel)Read this Medication Guide before you start your TECARTUS treatment. The more you know about your treatment, the more active you can be in your care. Talk with your healthcare provider if you have questions about your health condition or treatment. Reading this Medication Guide does not take the place of talking with your healthcare provider about your treatment. What is the most important information I should know about TECARTUS?
TECARTUS may cause side effects that are life-threatening and can lead to death. Call or see your healthcare provider or get emergency help right away if you get any of the following:- Fever (100.4°F/38°C or higher)
- Difficulty breathing
- Chills or shaking chills
- Confusion
- Dizziness or lightheadedness
- Severe nausea, vomiting, or diarrhea
- Fast or irregular heartbeat
- Severe fatigue or weakness
What is TECARTUS?
TECARTUS is a treatment for adults with mantle cell lymphoma or acute lymphoblastic leukemia. It is used following disease progression while on or after other treatment. TECARTUS is different than other cancer medicines because it is made from your own white blood cells, which have been modified to recognize and attack your lymphoma cells.Before getting TECARTUS, tell your healthcare provider about all your medical problems, including if you have or have had: - Neurologic problems (such as seizures, stroke, or memory loss)
- Lung or breathing problems
- Heart problems
- Liver problems
- Kidney problems
- A recent or active infection
How will I receive TECARTUS? - Since TECARTUS is made from your own white blood cells, your blood will be collected by a process called "leukapheresis" (loo-kah-fur-ee-sis), which will concentrate your white blood cells.
- Your blood cells will be sent to a manufacturing center to make your TECARTUS.
- Before you get TECARTUS, you will get three days of chemotherapy to prepare your body.
- When your TECARTUS is ready, your healthcare provider will give it to you through a catheter placed into your vein (intravenous infusion). The infusion usually takes less than 30 minutes.
- You will be monitored daily for at least 7 days after the infusion.
- You should plan to stay close to a healthcare facility for at least 2 weeks after getting TECARTUS. Your healthcare provider will help you with any side effects that may occur.
- You may be hospitalized for side effects. Your healthcare provider will discharge you if your side effects are under control and it is safe for you to leave the hospital.
- Your healthcare provider will want to do blood tests to follow your progress. It is important that you do have your blood tested. If you miss an appointment, call your healthcare provider as soon as possible to reschedule.
- Avoid driving for at least 2 weeks after you get TECARTUS.
- Do not donate blood, organs, tissues, or cells for transplantation.
What are the possible or reasonably likely side effects of TECARTUS?
The most common side effects of TECARTUS include:- Fever (100.4°F/38°C or higher)
- Low white blood cells (can occur with a fever)
- Low red blood cells
- Low blood pressure (dizziness or lightheadedness, headache, feeling tired, short of breath)
- Fast heartbeat
- Confusion
- Difficulty speaking or slurred speech
- Nausea
- Diarrhea
These are not all the possible side effects of TECARTUS. Call your healthcare provider about any side effects that concern you. You may report side effects to the FDA at 1-800-FDA-1088.General information about the safe and effective use of TECARTUS
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. If you would like more information about TECARTUS, talk with your healthcare provider. You can ask your healthcare provider for information about TECARTUS that is written for health professionals. You can get additional information by contacting Kite at 1-844-454-KITE (5483) or at www.Tecartus.com.What are the ingredients in TECARTUS?
Active ingredients: brexucabtagene autoleucel.
Inactive ingredients: albumin (human); DMSO.
TECARTUS is a trademark of Kite Pharma, Inc. All other trademarks referenced herein are the property of their respective owners.
©2025 Kite Pharma, Inc. All Rights Reserved. -
PRINCIPAL DISPLAY PANEL - 68 mL Bag Label - Product - MK-00379
NDC 71287-219-01
brexucabtagene autoleucel
TECARTUS®RX ONLY
FOR AUTOLOGOUS & INTRAVENOUS USE ONLY
No U.S. standard of potencyDose: One sterile bag for infusion.
Contents: Maximum of 2 x 108 autologous anti-CD19 CAR T cells in
approximately 68 mL suspension containing 5% DMSO USP.Gently mix the contents of the bag
while thawingSee package insert for full prescribing
information and instructions for
administrationShip and store in vapor phase of liquid
nitrogen ≤ -150°C. Optional one time
storage at -80°C as indicated in the
package insert.DO NOT USE A
LEUKODEPLETING FILTERDO NOT IRRADIATE
Manufactured with gentamicin
Not evaluated for infectious
substancesPreservative free
Manufacturer: Kite Pharma, Inc., 2400 Broadway, Santa Monica, CA 90404
Phone: 1-844-454-KITE U.S. Lic. #2064MK-00379

-
PRINCIPAL DISPLAY PANEL - 68 mL Cassette Label - Product - MK-00376
NDC 71287-219-02
brexucabtagene autoleucel
TECARTUS®RX ONLY
FOR AUTOLOGOUS & INTRAVENOUS USE ONLY
No U.S. standard of potencyDose: One sterile bag for infusion.
Contents: Maximum of 2 x 108 autologous anti-CD19 CAR T cells in
approximately 68 mL suspension containing 5% DMSO USP.Gently mix the contents of
the bag while thawingSee package insert for full
prescribing information and
instructions for administrationShip and store in vapor phase of
liquid nitrogen ≤ -150°C. Optional
one time storage at -80°C as
indicated in the package insert.DO NOT USE A
LEUKODEPLETING FILTERDO NOT IRRADIATE
Manufactured with gentamicin
Not evaluated for infectious
substancesPreservative free
Manufacturer: Kite Pharma, Inc., 2400 Broadway, Santa Monica, CA 90404
Phone: 1-844-454-KITE U.S. Lic. #2064Kite
A GILEAD CompanyMK-00376

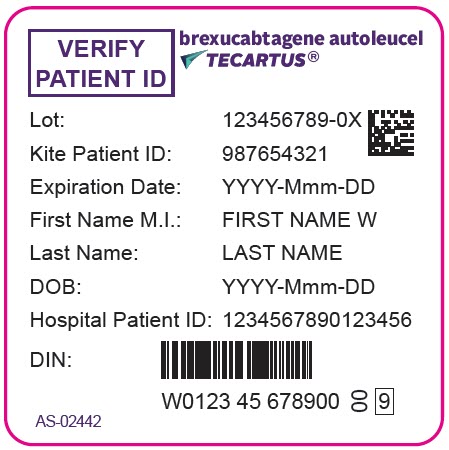
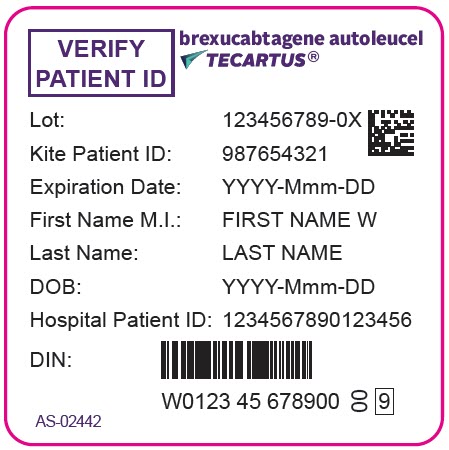
- PRINCIPAL DISPLAY PANEL - 68 mL Bag Label - Patient - AS-02442
-
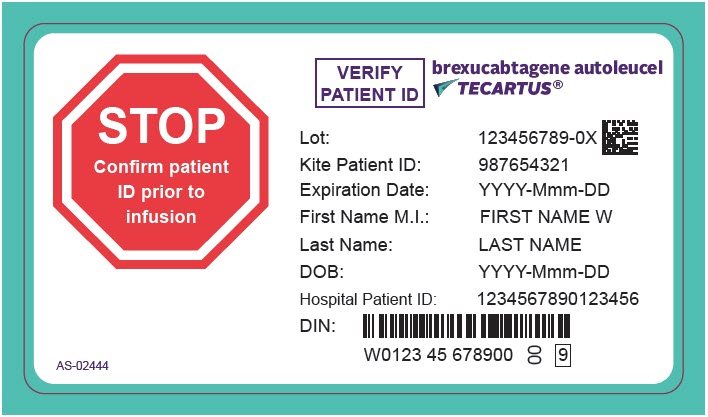
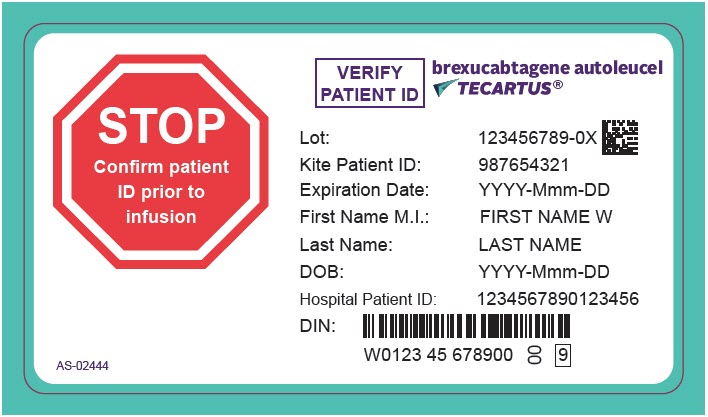
PRINCIPAL DISPLAY PANEL - 68 mL Cassette Label - Patient - AS-02444
STOP
Confirm patient
ID prior to
infusionVERIFY
PATIENT IDbrexucabtagene autoleucel
TECARTUS®Lot: 123456789-0X
Kite Patient ID: 987654321
Expiration Date: YYYY-Mmm-DD
First Name M.I.: FIRST NAME W
Last Name: LAST NAME
DOB: YYYY-Mmm-DD
Hospital Patient ID: 1234567890123456
DIN:
W0123 45 678900
00
9AS-02444
- PRINCIPAL DISPLAY PANEL - 68 mL LN2 Label - AS-02447
-
PRINCIPAL DISPLAY PANEL - 68 mL Bag Label - Product - MK-00380
NDC 71287-220-01
brexucabtagene autoleucel
TECARTUS®RX ONLY
FOR AUTOLOGOUS & INTRAVENOUS USE ONLY
No U.S. standard of potencyDose: One sterile bag for infusion.
Contents: Maximum of 1 x 108 autologous anti-CD19 CAR T cells in
approximately 68 mL suspension containing 5% DMSO USP.Gently mix the contents of the bag
while thawingSee package insert for full prescribing
information and instructions for
administrationShip and store in vapor phase of liquid
nitrogen ≤ -150°C. Optional one time
storage at -80°C as indicated in the
package insert.DO NOT USE A
LEUKODEPLETING FILTERDO NOT IRRADIATE
Manufactured with gentamicin
Not evaluated for infectious
substancesPreservative free
Manufacturer: Kite Pharma, Inc., 2400 Broadway, Santa Monica, CA 90404
Phone: 1-844-454-KITE U.S. Lic. #2064MK-00380

-
PRINCIPAL DISPLAY PANEL - 68 mL Cassette Label - Product - MK-00377
NDC 71287-220-02
brexucabtagene autoleucel
TECARTUS®RX ONLY
FOR AUTOLOGOUS & INTRAVENOUS USE ONLY
No U.S. standard of potencyDose: One sterile bag for infusion.
Contents: Maximum of 1 x 108 autologous anti-CD19 CAR T cells in
approximately 68 mL suspension containing 5% DMSO USP.Gently mix the contents of
the bag while thawingSee package insert for full
prescribing information and
instructions for administrationShip and store in vapor phase of
liquid nitrogen ≤ -150°C. Optional
one time storage at -80°C as
indicated in the package insert.DO NOT USE A
LEUKODEPLETING FILTERDO NOT IRRADIATE
Manufactured with gentamicin
Not evaluated for infectious
substancesPreservative free
Manufacturer: Kite Pharma, Inc., 2400 Broadway, Santa Monica, CA 90404
Phone: 1-844-454-KITE U.S. Lic. #2064Kite
A GILEAD CompanyMK-00377

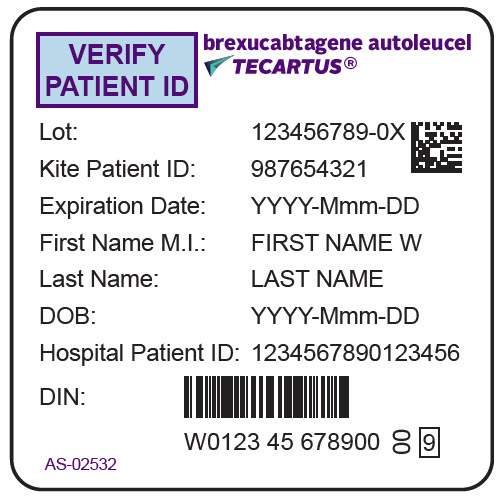
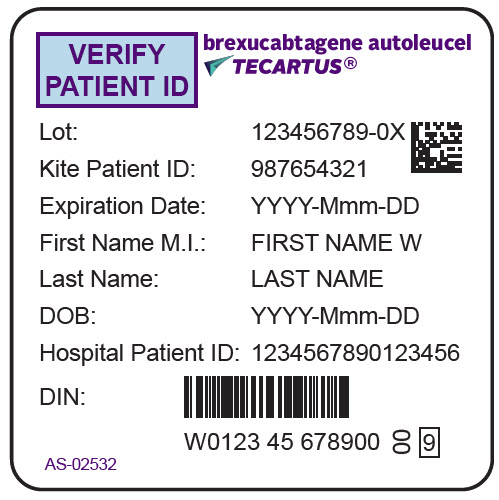
- PRINCIPAL DISPLAY PANEL - 68 mL Bag Label - Patient - AS-02532
-
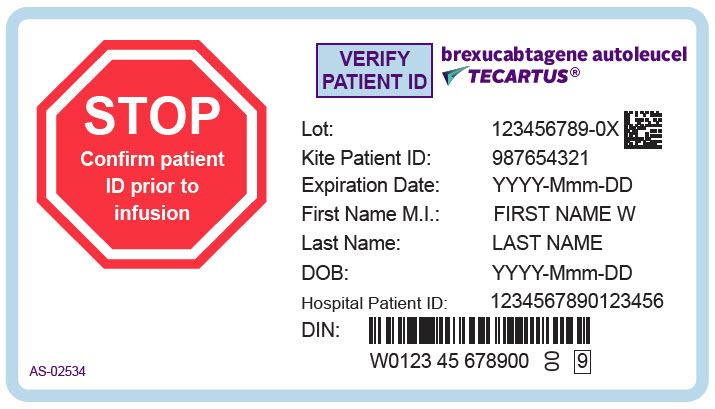
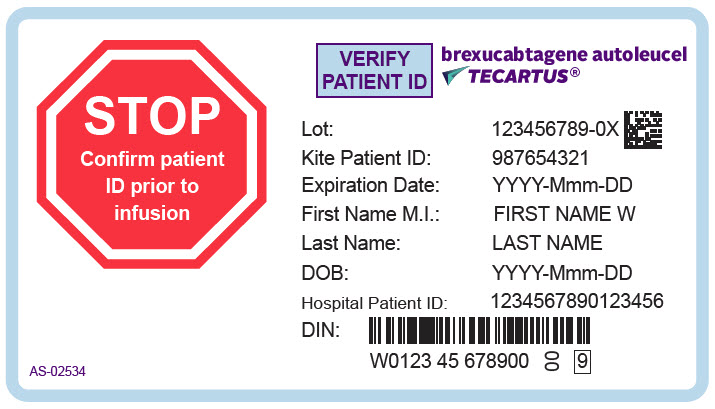
PRINCIPAL DISPLAY PANEL - 68 mL Cassette Label - Patient - AS-02534
STOP
Confirm patient
ID prior to
infusionVERIFY
PATIENT IDbrexucabtagene autoleucel
TECARTUS®Lot: 123456789-0X
Kite Patient ID: 987654321
Expiration Date: YYYY-Mmm-DD
First Name M.I.: FIRST NAME W
Last Name: LAST NAME
DOB: YYYY-Mmm-DD
Hospital Patient ID: 1234567890123456
DIN:
W0123 45 678900
00
9AS-02534
- PRINCIPAL DISPLAY PANEL - 68 mL LN2 Label - AS-02536
-
INGREDIENTS AND APPEARANCE
TECARTUS
brexucabtagene autoleucel suspensionProduct Information Product Type CELLULAR THERAPY Item Code (Source) NDC:71287-219 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BREXUCABTAGENE AUTOLEUCEL (UNII: 4MD2J2T8SJ) (BREXUCABTAGENE AUTOLEUCEL - UNII:4MD2J2T8SJ) BREXUCABTAGENE AUTOLEUCEL 2000000 in 68 mL Inactive Ingredients Ingredient Name Strength DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71287-219-02 1 in 1 PACKAGE 1 NDC:71287-219-01 68 mL in 1 BAG; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125703 07/24/2020 TECARTUS
brexucabtagene autoleucel suspensionProduct Information Product Type CELLULAR THERAPY Item Code (Source) NDC:71287-220 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BREXUCABTAGENE AUTOLEUCEL (UNII: 4MD2J2T8SJ) (BREXUCABTAGENE AUTOLEUCEL - UNII:4MD2J2T8SJ) BREXUCABTAGENE AUTOLEUCEL 1000000 in 68 mL Inactive Ingredients Ingredient Name Strength DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71287-220-02 1 in 1 PACKAGE 1 NDC:71287-220-01 68 mL in 1 BAG; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125703 10/01/2021 Labeler - Kite Pharma, Inc. (963353359) Establishment Name Address ID/FEI Business Operations KITE PHARMA, INC. 963353359 MANUFACTURE(71287-219) , LABEL(71287-219) Establishment Name Address ID/FEI Business Operations Kite Pharma, Inc. 116931311 ANALYSIS(71287-219)