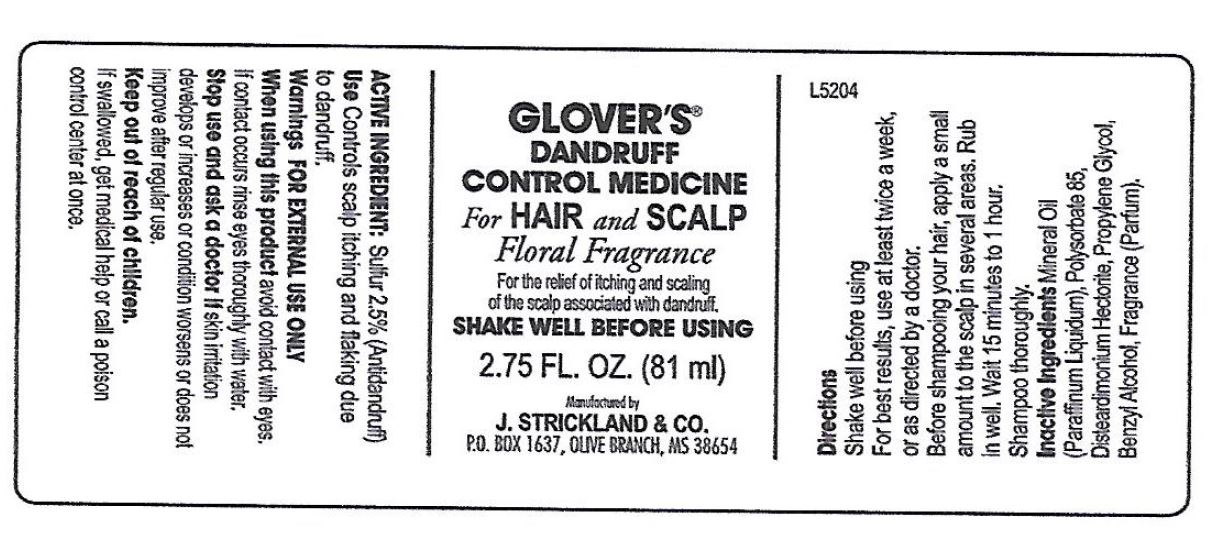
Label: GLOVERS DANDRUFF CONTROL MED., FLORAL- sulfur suspension
- NDC Code(s): 12022-008-00
- Packager: J. Strickland & Co.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 21, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients:
- Uses:
- Warnings:
- Directions:
- Inactive Ingredients:
- Package Labeling Bottle
- Package Labeling Carton
-
INGREDIENTS AND APPEARANCE
GLOVERS DANDRUFF CONTROL MED., FLORAL
sulfur suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:12022-008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) POLYSORBATE 85 (UNII: A7F3N56197) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12022-008-00 1 in 1 CARTON 11/05/2001 1 81 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 11/05/2001 Labeler - J. Strickland & Co. (007023112) Registrant - J. Strickland & Co. (007023112) Establishment Name Address ID/FEI Business Operations J. Strickland & Co. 007023112 manufacture(12022-008)