Label: ULTRA STRENGTH ANTACID ASSORTED FRUIT- calcium carbonate tablet, chewable
-
Contains inactivated NDC Code(s)
NDC Code(s): 49035-169-16 - Packager: Wal-Mart Stores,Inc.,
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 4, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
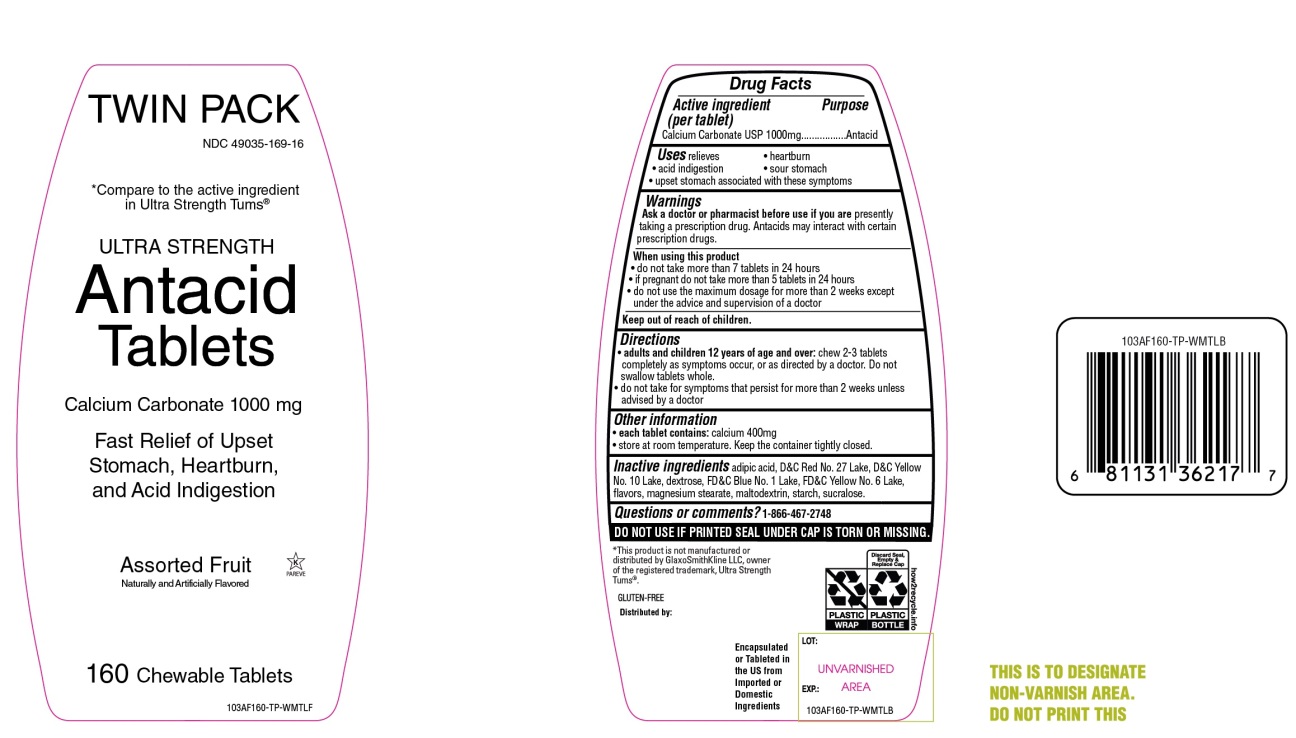
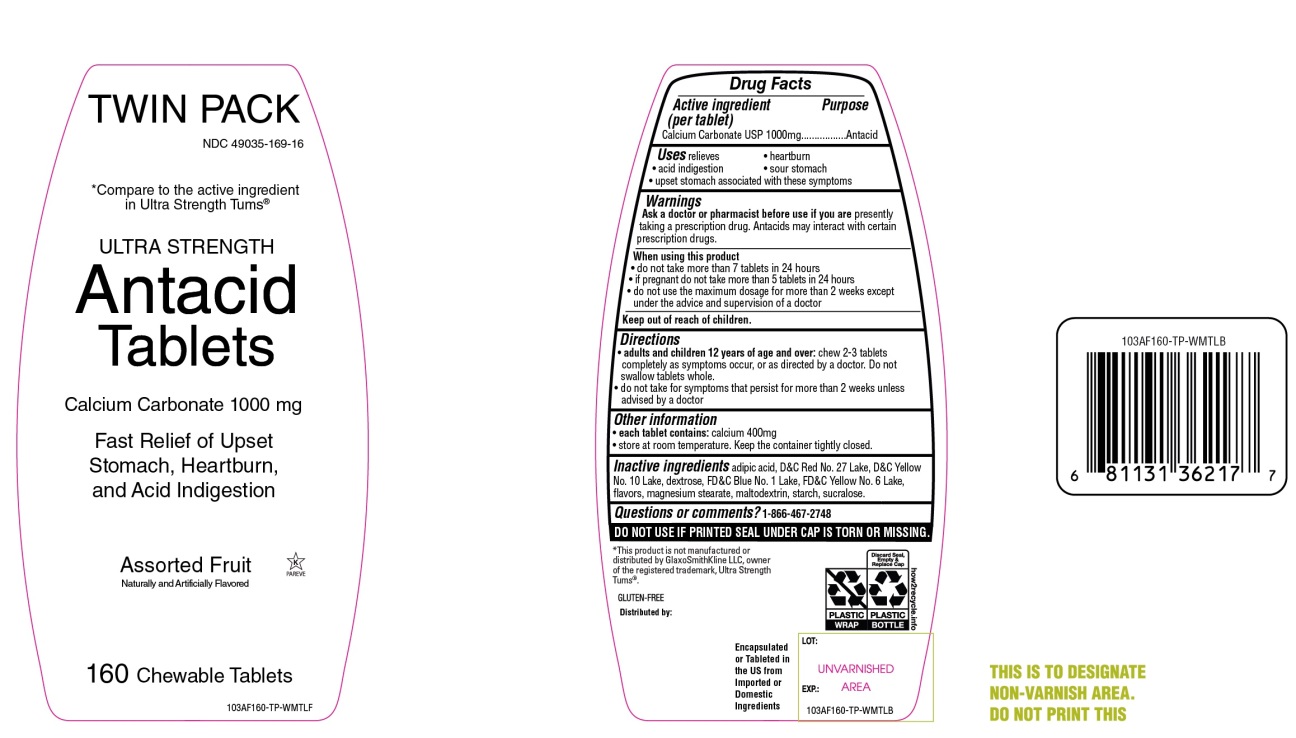
- Active ingredient (per tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor or pharmacist before use if you are presently taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product
- •
- do not take more than 7 tablets in 24 hours
- •
- if pregnant do not take more than 5 tablets in 24 hours
- •
- do not use the maximum dosage for more than 2 weeks except under the advice and supervision of a doctor.
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
-
Package/Label Principal Display Panel
NDC 49035-169-16
equate™
Compare to the active ingredient in Ultra Strength Tums®
ULTRA STRENGTH
Antacid Tablets
Calcium Carbonate 1000 mg
Fast Relief of Upset Stomach, Heartburn and Acid Indigestion
Assorted Fruit Flavors
Naturally and Artificially Flavored
160 CHEWABLE TABLETS
Gluten- Free
Satisfaction guaranteed-
For questions or comments, please call
1-888-287-1915.
Distributed by: Wal-Mart Stores, Inc.,
Bentonville,AR 72716
*This product is not manufactured or distributed by GlaxoSmithKline LLC, owner of the registered trademark, Tums®.
-
INGREDIENTS AND APPEARANCE
ULTRA STRENGTH ANTACID ASSORTED FRUIT
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49035-169 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CARBONATE ION - UNII:7UJQ5OPE7D, CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 1000 mg Inactive Ingredients Ingredient Name Strength ADIPIC ACID (UNII: 76A0JE0FKJ) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) STARCH, CORN (UNII: O8232NY3SJ) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color RED, GREEN, YELLOW, ORANGE Score no score Shape ROUND Size 19mm Flavor FRUIT (assorted fruit) Imprint Code RP103 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49035-169-16 160 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/26/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 11/25/2019 Labeler - Wal-Mart Stores,Inc., (051957769)