Label: NARCAN- naloxone hydrochloride nasal spray
- NDC Code(s): 69547-627-02
- Packager: Emergent Devices Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each spray)
- Purpose
- Uses
- Warning
-
Directions
NARCAN®
Naloxone HCl Nasal Spray 4 mgStep 1: CHECK if you suspect an overdose:
- •
-
CHECK for a suspected overdose: the person will not wake up or is very sleepy or not breathing well
- •
- yell “Wake up!”
- •
- shake the person gently
- •
- if the person is not awake, go to Step 2
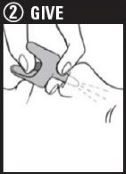
Step 2: Give 1st dose in the nose
- •
- HOLD the nasal spray device with your thumb on the bottom of the plunger
- •
- INSERT the nozzle into either NOSTRIL
- •
- PRESS the plunger firmly to give the 1st dose
- •
- 1 nasal spray device contains 1 dose
Step 3: CALL 911
- •
- CALL 911 immediately after giving the lst dose
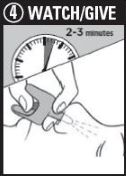
Step 4: WATCH & GIVE
- •
- WAIT 2-3 minutes after the lst dose to give the medicine time to work
- •
- if the person wakes up: Go to Step 5
- •
- if the person does not wake up:
- •
- CONTINUE TO GIVE doses every 2-3 minutes until the person wakes up
- •
- it is safe to keep giving doses
Step 5: STAY
- •
- STAY until ambulance arrives: even if the person wakes up
- •
- GIVE another dose if the person becomes very sleepy again
- •
- You may need to give all the doses in the pack
For opioid emergencies, call 911. For questions on NARCAN, call 1-844-4NARCAN (1-844-462-7226) or go to www.narcan.com.
©2023 Emergent Devices Inc. EMERGENT® and NARCAN® are registered trademarks of Emergent BioSolutions Inc, or its subsidiaries.
Other Information
- •
- store at room temperature or refrigerated, between 2°C to 25°C (36°F to 77°F)
- •
- do not freeze
- •
- avoid excessive heat above 40°C (104°F)
- •
- protect from light
- •
- the product is packaged in individually-sealed blisters.
Do not use if the blister is open or torn, or if the device appears damaged.
- Inactive Ingredients
- Questions?
-
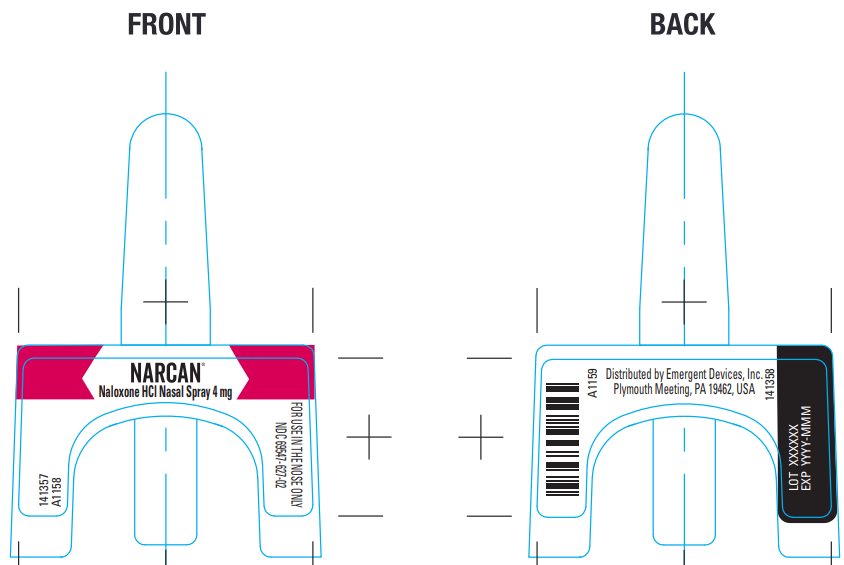
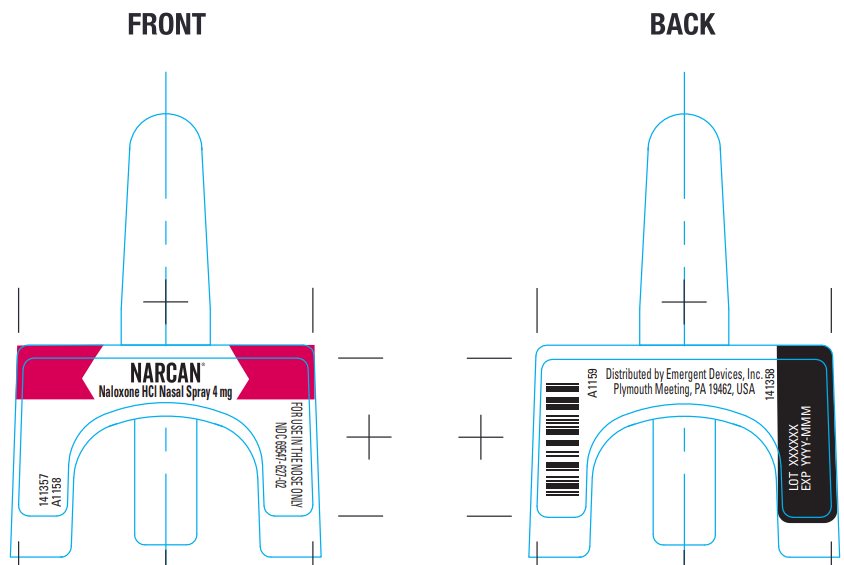
Package/Label Principal Display Panel – Carton Label
NARCAN®
Naloxone HCl Nasal Spray 4 mg
Emergency Treatment of Opioid OverdoseOriginal
Prescription
StrengthEasy to Use
Can Save a Life
Designed to
Rapidly Reverse
the Effects of a
Life-Threatening
Opioid Emergency2 Single-Dose Nasal Spray Devices
0.003 fl oz (0.1 mL) eachFor use in
nose onlyDistributed by Emergent Devices Inc.
Plymouth Meeting, PA 19462 USA©2023 Emergent Devices Inc. Plymouth Meeting, PA.
All rights reserved.NARCAN® is a registered trademark of Emergent Operations
Ireland Limited.NDC 69547-627-02
-
Package/Label Principal Display Panel - 4 mg Vial Package
NDC 69547-627-02
NARCAN®
Naloxone HCl Nasal Spray 4 mg
Emergency Treatment of Opioid Overdose- •
- For use in the nose only
- •
- Do not test nasal spray device before use
- •
- 1 nasal spray device contains 1 dose of medicine
- •
- Each device sprays 1 time only
- •
- Store at room temperature or refrigerated,
between 2°C to 25°C (36°F to 77°F) - •
- Do not freeze
- •
- Avoid excessive heat above 40°C (104°F)
Lot XXXXXX EXP YYYY-MMM
Distributed by Emergent Devices Inc.
Plymouth Meeting, PA 19462 USA A1160 - Package/Label Principal Display Panel – Front and Back Label
-
INGREDIENTS AND APPEARANCE
NARCAN
naloxone hydrochloride nasal sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69547-627 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE HYDROCHLORIDE 4 mg in 0.1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69547-627-02 2 in 1 CARTON 08/28/2023 1 0.1 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208411 08/28/2023 Labeler - Emergent Devices Inc. (079673287)