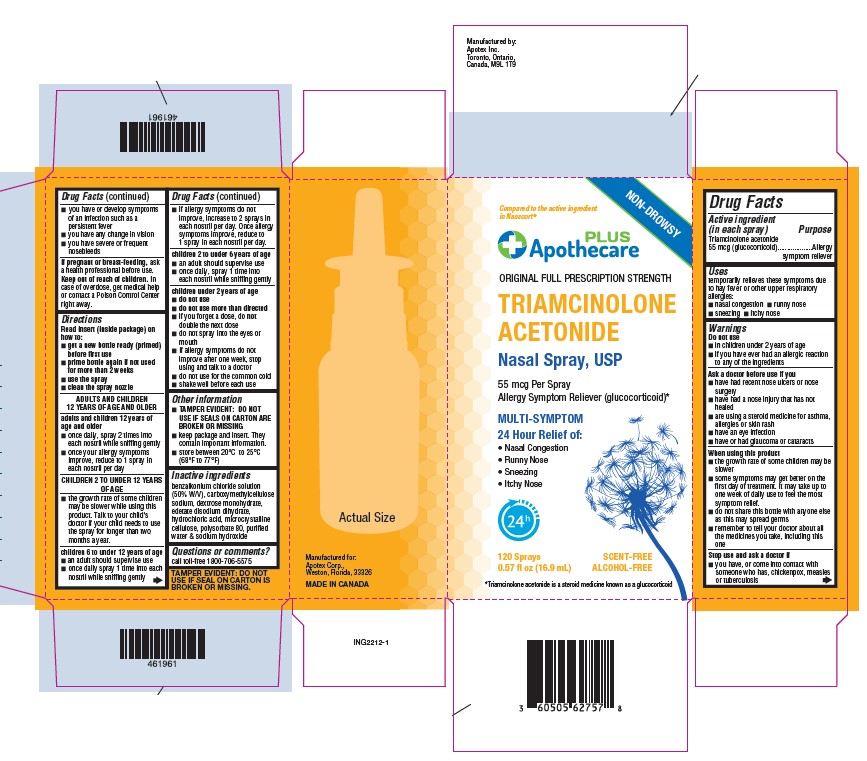
Label: TRIAMCINOLONE ACETONIDE spray, metered
- NDC Code(s): 60505-6275-7
- Packager: Apotex Corp.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each spray)
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- In children under 2 years of age
- If you have ever had an allergic reaction to any of the ingredients
Ask a doctor before use if you
- have had recent nose ulcers or nose surgery
- have had a nose injury that has not healed
- are using a steroid medicine for asthma, allergies or skin rash
- have an eye infection
- have or had glaucoma or cataracts
When using this product
- the growth rate of some children may be slower
- some symptoms may get better on the first day of treatment. It may take up to one week of daily use to feel the most symptom relief.
- do not share this bottle with anyone else as this may spread germs
- remember to tell your doctor about all the medicines you take, including this one
-
Directions
Read insert (inside package) on how to:
- get a new bottle ready (primed) before first use
- prime bottle again if not used for more than 2 weeks
- use the spray
-
clean the spray nozzle
ADULTS AND CHILDREN 12 YEARS OF AGE AND OLDER adults and children 12 years of age and older - once daily, spray 2 times into each nostril while sniffing gently
- once your allergy symptoms improve, reduce to 1 spray in each nostril
CHILDREN 2 TO UNDER 12 YEARS OF AGE - the growth rate of some children may be slower while using this product. Talk to your child’s doctor if your child needs to use the spray longer than two months a year.
children 6 to under 12 years of age - an adult should supervise use
- once daily, spray 1 time into each nostril while sniffing gently
- if allergy symptoms do not improve, increase to 2 sprays in each nostril per day. Once allergy symptoms improve, reduce to 1 spray in each nostril per day.
children 2 to under 6 years of age - an adult should supervise use
- once daily, spray 1 time into each nostril while sniffing gently
children under 2 years of age - do not use
- do not use more than directed
- if you forget a dose, do not double the next dose
- do not spray into eyes or mouth
- if allergy symptoms do not improve after one week, stop using and talk to a doctor
- do not use for the common cold
- shake well before each use
- Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
TRIAMCINOLONE ACETONIDE
triamcinolone acetonide spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60505-6275 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIAMCINOLONE ACETONIDE (UNII: F446C597KA) (Triamcinolone Acetonide - UNII:F446C597KA) TRIAMCINOLONE ACETONIDE 55 ug Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60505-6275-7 1 in 1 CARTON 01/26/2024 1 120 in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA214615 01/26/2024 Labeler - Apotex Corp. (845263701)