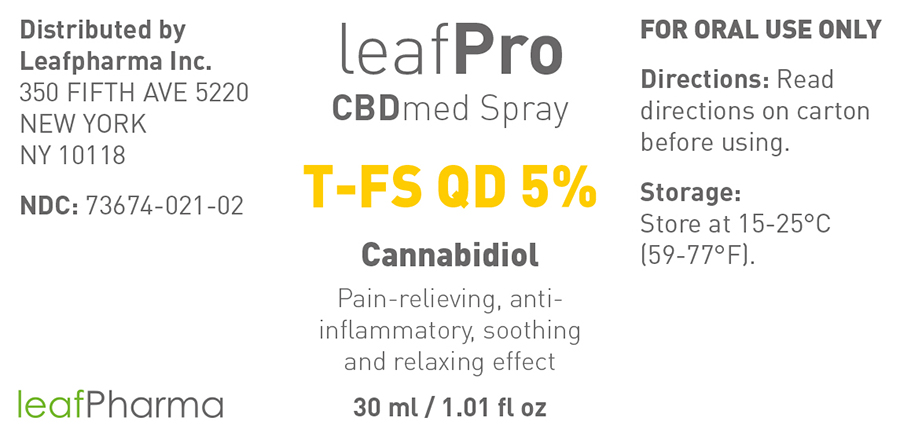
Label: LEAFPRO CBDMED OIL T-FS QD 5%- cannabidiol oil
-
Contains inactivated NDC Code(s)
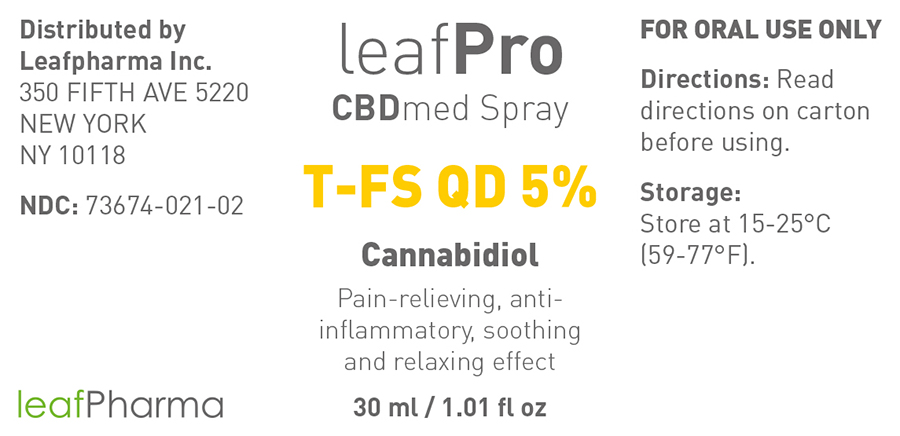
NDC Code(s): 73674-021-01, 73674-021-02 - Packager: leafMed GmbH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: CV
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 13, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
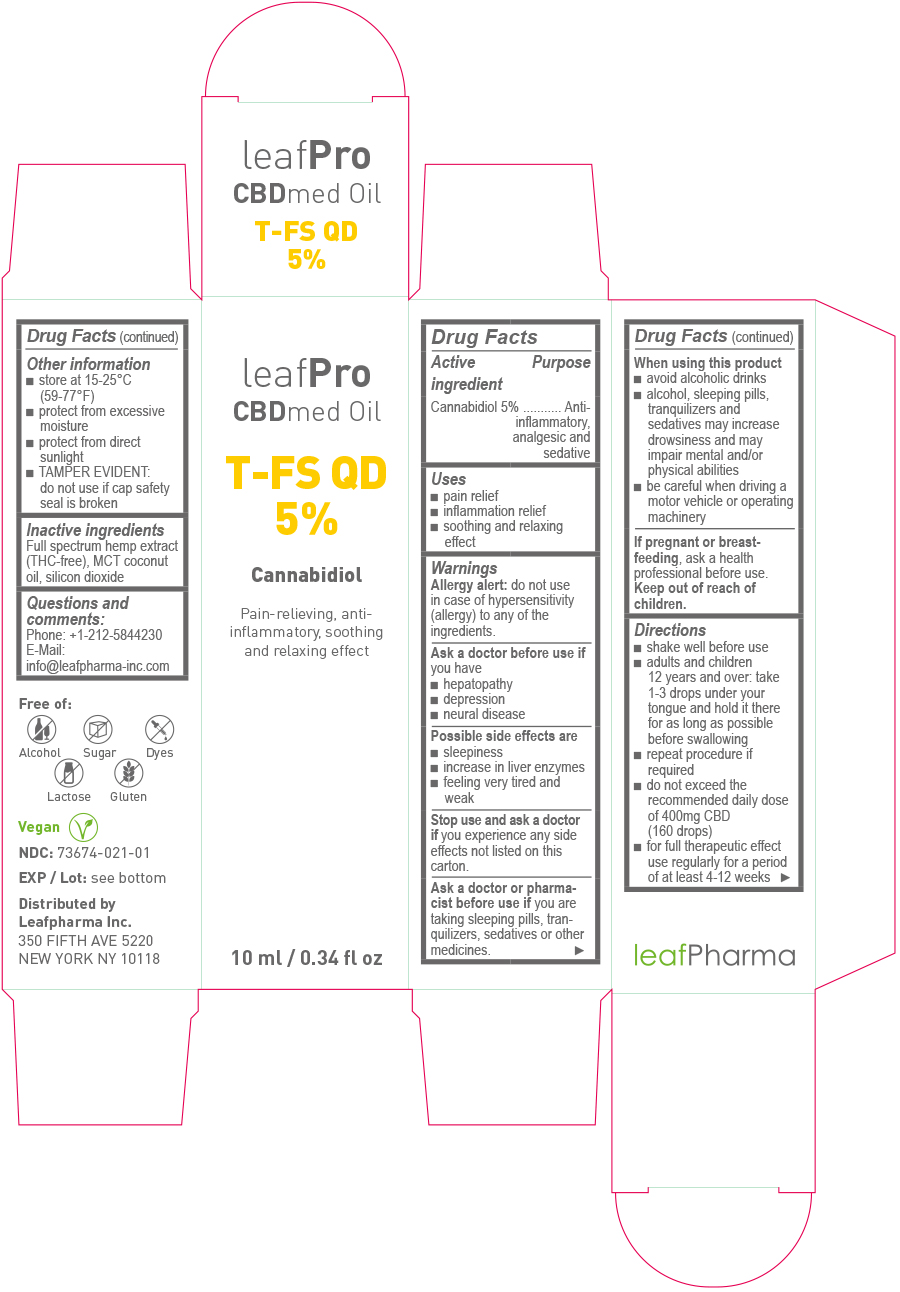
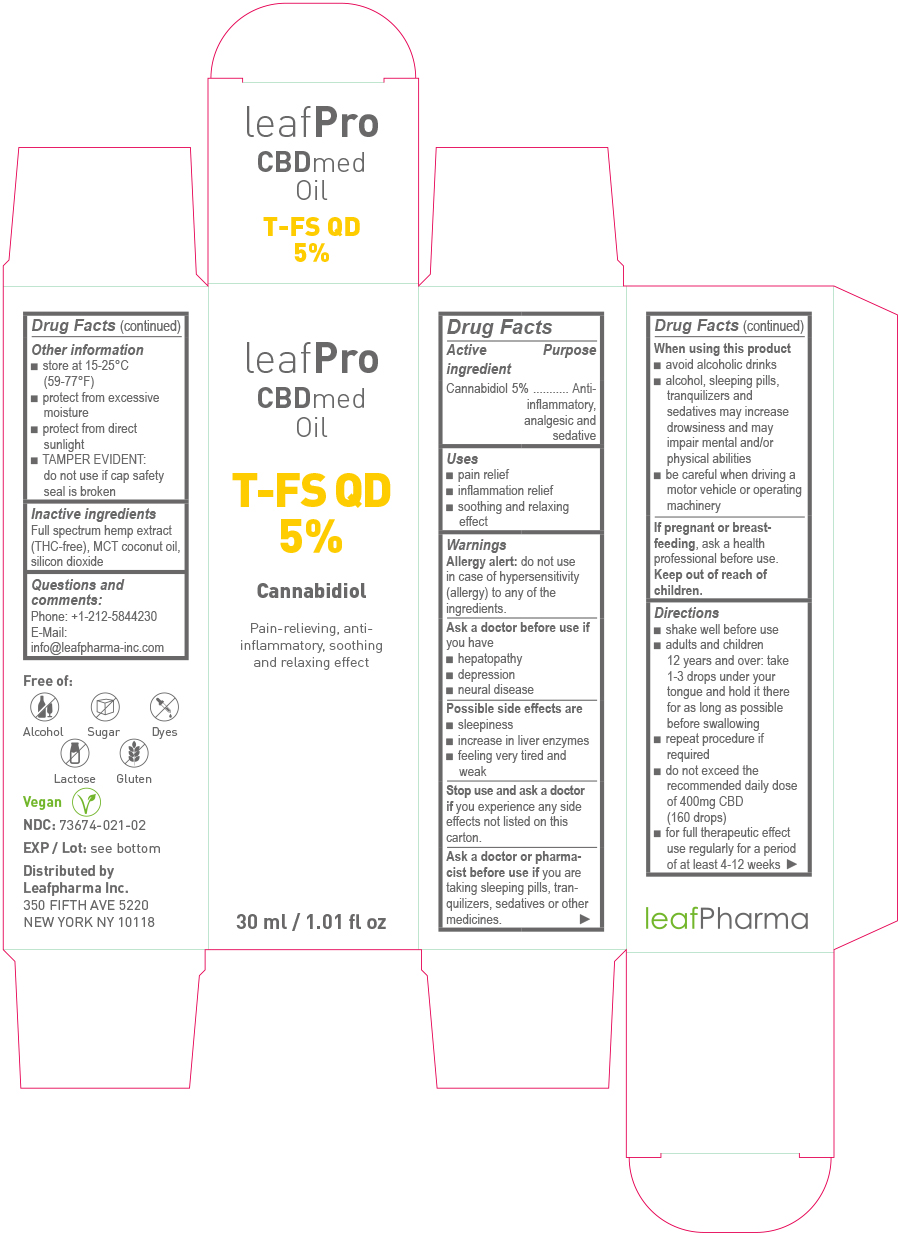
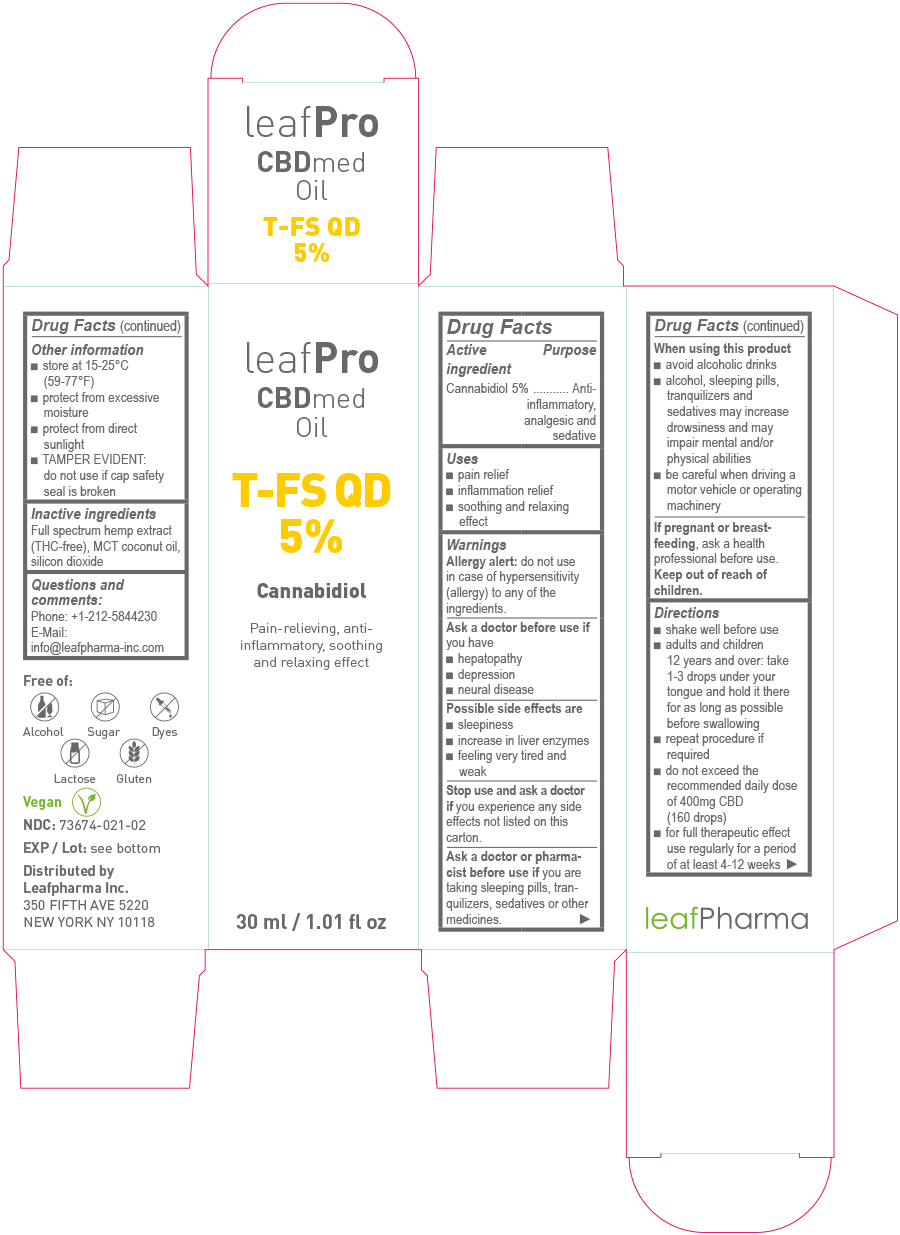
- Drug Facts
- Active Ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor or pharmacist before use if
you are taking sleeping pills, tranquilizers, sedatives or other medicines.
-
Directions
- shake well before use
- adults and children 12 years and over: take 1 – 3 drops under your tongue and hold it there for as long as possible before swallowing
- repeat procedure if required
- do not exceed the recommended daily dose of 400mg CBD (160 drops)
- for full therapeutic effect use regularly for a period of at least 4-12 weeks
- Other information
- Inactive ingredients:
- Questions and comments:
- Principal Display Panel - 10ml
- Principal Display Panel - 30ml
-
INGREDIENTS AND APPEARANCE
LEAFPRO CBDMED OIL T-FS QD 5%
cannabidiol oilProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73674-021 Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CANNABIDIOL (UNII: 19GBJ60SN5) (CANNABIDIOL - UNII:19GBJ60SN5) CANNABIDIOL 5 g in 100 mL Inactive Ingredients Ingredient Name Strength HEMP (UNII: TD1MUT01Q7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73674-021-01 1 in 1 CARTON 04/01/2020 1 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC:73674-021-02 1 in 1 CARTON 04/01/2020 2 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/01/2020 Labeler - leafMed GmbH (342895093) Registrant - Leafpharma Inc. (117330180) Establishment Name Address ID/FEI Business Operations leafMed GmbH 342895093 manufacture(73674-021) , pack(73674-021) , label(73674-021)