Label: PALIPERIDONE PALMITATE kit
-
NDC Code(s):
63646-700-56,
63646-701-39,
63646-702-78,
63646-703-17, view more63646-704-34, 63646-710-56, 63646-711-39, 63646-712-78, 63646-713-17, 63646-714-34
- Packager: TOLMAR Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated January 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
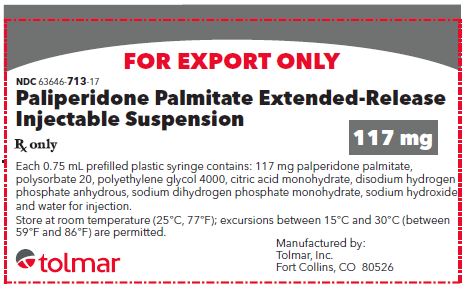
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PALIPERIDONE PALMITATE
paliperidone palmitate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63646-711 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63646-711-39 1 in 1 CARTON; Type 0: Not a Combination Product 01/01/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE 0.25 mL Part 1 of 1 PALIPERIDONE PALMITATE
paliperidone palmitate injection, suspension, extended releaseProduct Information Item Code (Source) NDC:63646-701 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PALIPERIDONE PALMITATE (UNII: R8P8USM8FR) (PALIPERIDONE - UNII:838F01T721) PALIPERIDONE PALMITATE 39 mg in 0.25 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 20 (UNII: 7T1F30V5YH) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63646-701-39 0.25 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/01/2022 PALIPERIDONE PALMITATE
paliperidone palmitate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63646-712 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63646-712-78 1 in 1 CARTON; Type 0: Not a Combination Product 01/01/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE 0.5 mL Part 1 of 1 PALIPERIDONE PALMITATE
paliperidone palmitate injection, suspension, extended releaseProduct Information Item Code (Source) NDC:63646-702 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PALIPERIDONE PALMITATE (UNII: R8P8USM8FR) (PALIPERIDONE - UNII:838F01T721) PALIPERIDONE PALMITATE 78 mg in 0.5 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 20 (UNII: 7T1F30V5YH) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63646-702-78 0.5 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/01/2022 PALIPERIDONE PALMITATE
paliperidone palmitate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63646-713 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63646-713-17 1 in 1 CARTON; Type 0: Not a Combination Product 01/01/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE 0.75 mL Part 1 of 1 PALIPERIDONE PALMITATE
paliperidone palmitate injection, suspension, extended releaseProduct Information Item Code (Source) NDC:63646-703 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PALIPERIDONE PALMITATE (UNII: R8P8USM8FR) (PALIPERIDONE - UNII:838F01T721) PALIPERIDONE PALMITATE 117 mg in 0.75 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 20 (UNII: 7T1F30V5YH) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63646-703-17 0.75 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/01/2022 PALIPERIDONE PALMITATE
paliperidone palmitate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63646-710 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63646-710-56 1 in 1 CARTON; Type 0: Not a Combination Product 01/01/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE 1 mL Part 1 of 1 PALIPERIDONE PALMITATE
paliperidone palmitate injection, suspension, extended releaseProduct Information Item Code (Source) NDC:63646-700 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PALIPERIDONE PALMITATE (UNII: R8P8USM8FR) (PALIPERIDONE - UNII:838F01T721) PALIPERIDONE PALMITATE 156 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 20 (UNII: 7T1F30V5YH) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63646-700-56 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/01/2022 PALIPERIDONE PALMITATE
paliperidone palmitate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63646-714 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63646-714-34 1 in 1 CARTON; Type 0: Not a Combination Product 01/01/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE 1.5 mL Part 1 of 1 PALIPERIDONE PALMITATE
paliperidone palmitate injection, suspension, extended releaseProduct Information Item Code (Source) NDC:63646-704 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PALIPERIDONE PALMITATE (UNII: R8P8USM8FR) (PALIPERIDONE - UNII:838F01T721) PALIPERIDONE PALMITATE 234 mg in 1.5 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 20 (UNII: 7T1F30V5YH) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63646-704-34 1.5 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/01/2022 Labeler - TOLMAR Inc. (791156578) Establishment Name Address ID/FEI Business Operations TOLMAR, INC. 079112310 analysis(63646-711, 63646-701, 63646-712, 63646-702, 63646-713, 63646-703, 63646-710, 63646-700, 63646-714, 63646-704) , label(63646-711, 63646-701, 63646-712, 63646-702, 63646-713, 63646-703, 63646-710, 63646-700, 63646-714, 63646-704) , manufacture(63646-711, 63646-701, 63646-712, 63646-702, 63646-713, 63646-703, 63646-710, 63646-700, 63646-714, 63646-704) , pack(63646-711, 63646-701, 63646-712, 63646-702, 63646-713, 63646-703, 63646-710, 63646-700, 63646-714, 63646-704)