Label: FRESH SKIN ORGANICS- ab nat zinc lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 58418-425-00, 58418-425-01, 58418-425-02, 58418-425-04, view more58418-425-05, 58418-425-08, 58418-425-09, 58418-425-12, 58418-425-16, 58418-425-28, 58418-425-34, 58418-425-55, 58418-425-64 - Packager: Tropical Enterprises International, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 8, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
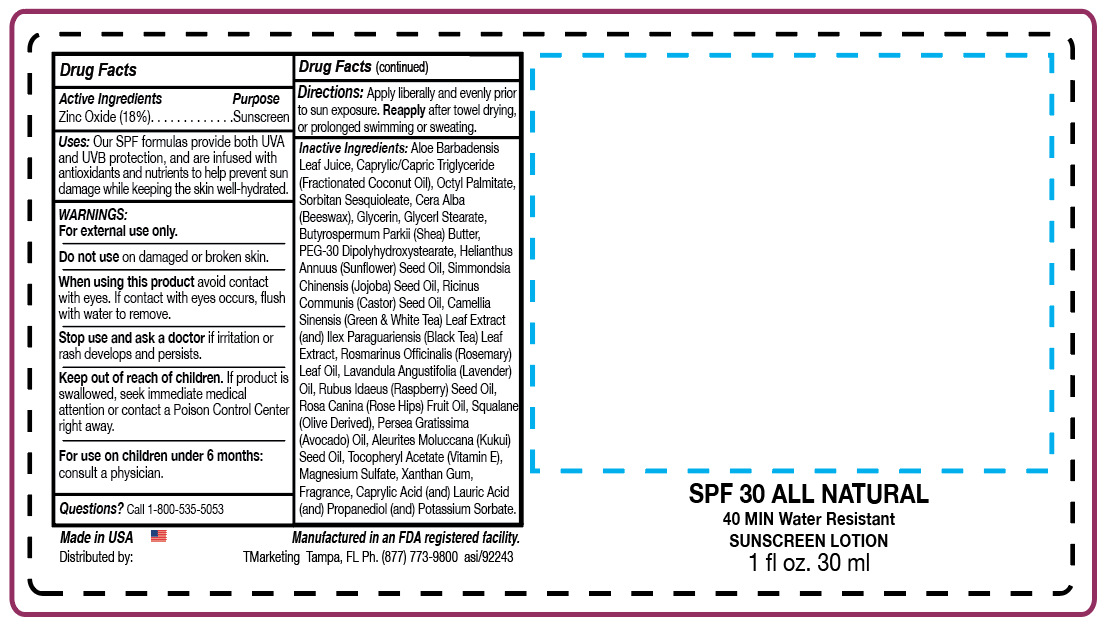
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FRESH SKIN ORGANICS
ab nat zinc lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58418-425 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.05 g in 30 mL Inactive Ingredients Ingredient Name Strength POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PROPANEDIOL (UNII: 5965N8W85T) CASTOR OIL (UNII: D5340Y2I9G) ROSEMARY OIL (UNII: 8LGU7VM393) LAVENDER OIL (UNII: ZBP1YXW0H8) KUKUI NUT OIL (UNII: TP11QR7B8R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ETHYLHEXYL PALMITATE (UNII: 2865993309) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) GLYCERIN (UNII: PDC6A3C0OX) SHEA BUTTER (UNII: K49155WL9Y) JOJOBA OIL (UNII: 724GKU717M) YELLOW WAX (UNII: 2ZA36H0S2V) LAURIC ACID (UNII: 1160N9NU9U) CAPRYLIC/CAPRIC/LAURIC TRIGLYCERIDE (UNII: FJ1H6M2JG9) AVOCADO OIL (UNII: 6VNO72PFC1) XANTHAN GUM (UNII: TTV12P4NEE) PEG-30 DIPOLYHYDROXYSTEARATE (UNII: 9713Q0S7FO) ROSA CANINA FRUIT OIL (UNII: CR7307M3QZ) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) CAPRYLIC ACID (UNII: OBL58JN025) SUNFLOWER OIL (UNII: 3W1JG795YI) CAMELLIA SINENSIS WHOLE (UNII: C5M4585ZBZ) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) RASPBERRY SEED OIL (UNII: 9S8867952A) SQUALANE (UNII: GW89575KF9) Product Characteristics Color white (zinc lotion) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58418-425-00 15 mL in 1 PACKET; Type 0: Not a Combination Product 03/02/2020 2 NDC:58418-425-05 15 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 03/02/2020 3 NDC:58418-425-01 30 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 03/02/2020 4 NDC:58418-425-02 60 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 03/02/2020 5 NDC:58418-425-04 120 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 03/02/2020 6 NDC:58418-425-08 240 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 03/02/2020 7 NDC:58418-425-09 240 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 03/02/2020 8 NDC:58418-425-16 480 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 03/02/2020 9 NDC:58418-425-12 360 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 03/02/2020 10 NDC:58418-425-64 1920 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 03/02/2020 11 NDC:58418-425-34 100.55 mL in 1 TUBE; Type 0: Not a Combination Product 03/01/2020 12 NDC:58418-425-28 3840 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 03/02/2020 13 NDC:58418-425-55 211200 mL in 1 DRUM; Type 0: Not a Combination Product 03/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/01/2020 Labeler - Tropical Enterprises International, Inc. (091986179) Registrant - Tropical Enterprises International, Inc. (091986179) Establishment Name Address ID/FEI Business Operations Tropical Enterprises International, Inc. 091986179 repack(58418-425)