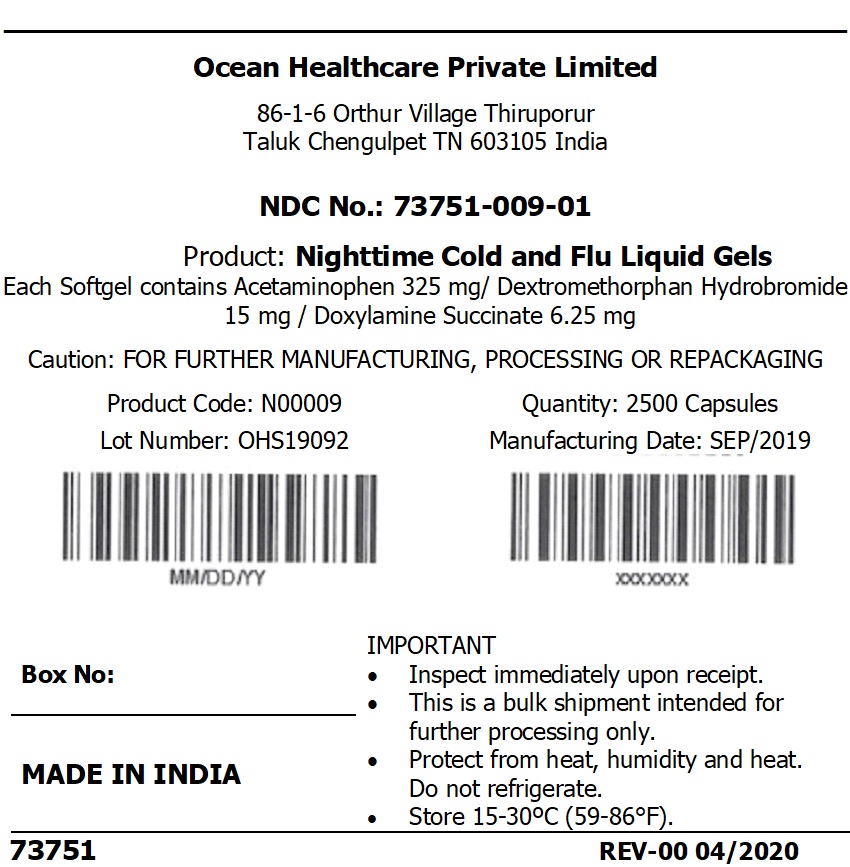
Label: NIGHTTIME COLD AND FLU LIQUID GELS capsule, liquid filled
DAYTIME COLD AND FLU LIQUID GELS capsule, liquid filled
- NDC Code(s): 73751-003-01, 73751-009-01
- Packager: Ocean Healthcare Private Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated November 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
-
DO NOT USE
Do not use
- With any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- PURPOSE
- QUESTIONS
-
STOP USE
Stop use and ask a doctor if
- You get nervous, dizzy or sleepless
- Symptoms get worse or last more than 5 days (children) or 7 days (adults)
- Fever gets worse or lasts more than 3 days
- Redness or swelling is present
- New symptoms occur
- Cough comes back, or occurs with rash or headache that lasts. These could be signs of a serious condition.
- WHEN USING
- ACTIVE INGREDIENT
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
-
DO NOT USE
Do not use
- Wth any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- To make a child sleep
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- PURPOSE
- QUESTIONS
- STOP USE
- WHEN USING
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NIGHTTIME COLD AND FLU LIQUID GELS
nighttime cold and flu liquid gels capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73751-009 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 15 mg DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 6.25 mg Inactive Ingredients Ingredient Name Strength SORBITOL SOLUTION 70% (UNII: 8KW3E207O2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POVIDONE (UNII: FZ989GH94E) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color green Score no score Shape OVAL (Oblong Shape) Size 21mm Flavor Imprint Code AP02 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73751-009-01 2500 in 1 BAG; Type 0: Not a Combination Product 04/06/2020 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 04/06/2020 DAYTIME COLD AND FLU LIQUID GELS
daytime cold and flu liquid gels capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73751-003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) SORBITOL SOLUTION 70% (UNII: 8KW3E207O2) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color red Score no score Shape OVAL (Oblong Shaped) Size 21mm Flavor Imprint Code AP01 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73751-003-01 2500 in 1 BAG; Type 0: Not a Combination Product 04/06/2020 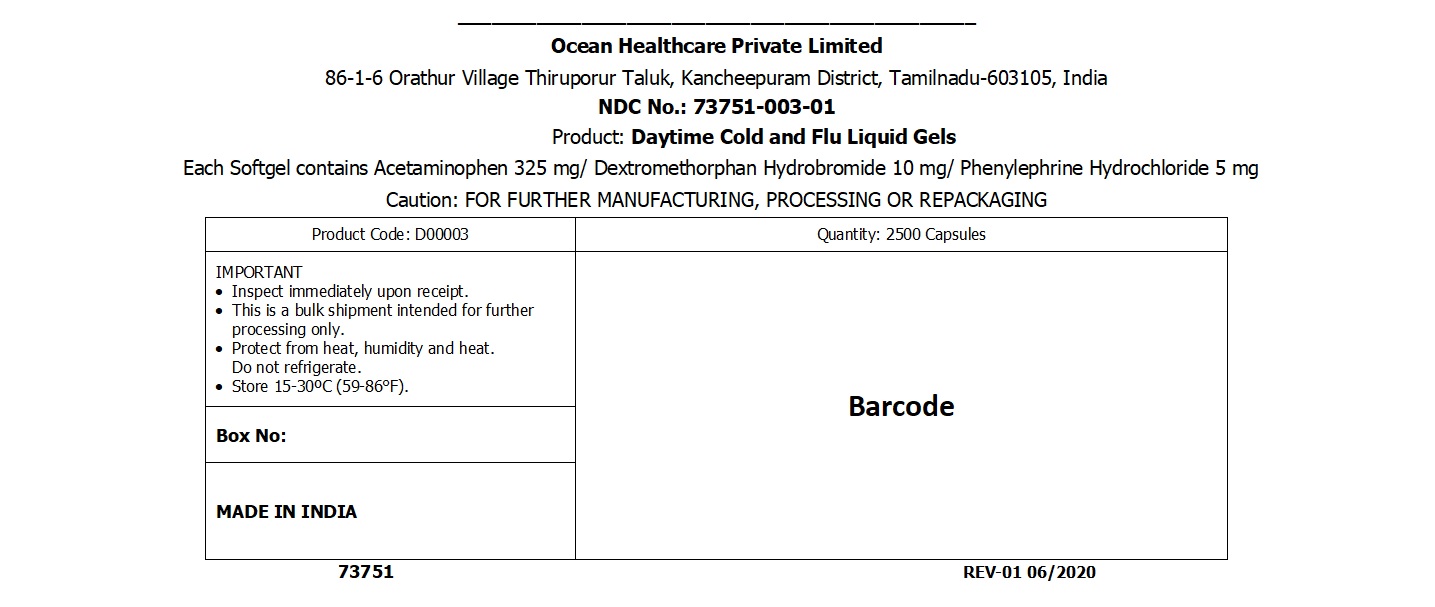
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 04/06/2020 Labeler - Ocean Healthcare Private Limited (873673519) Registrant - Sidharth Baid (873673519) Establishment Name Address ID/FEI Business Operations Ocean Healthcare Private Limited 873673519 manufacture(73751-003, 73751-009)