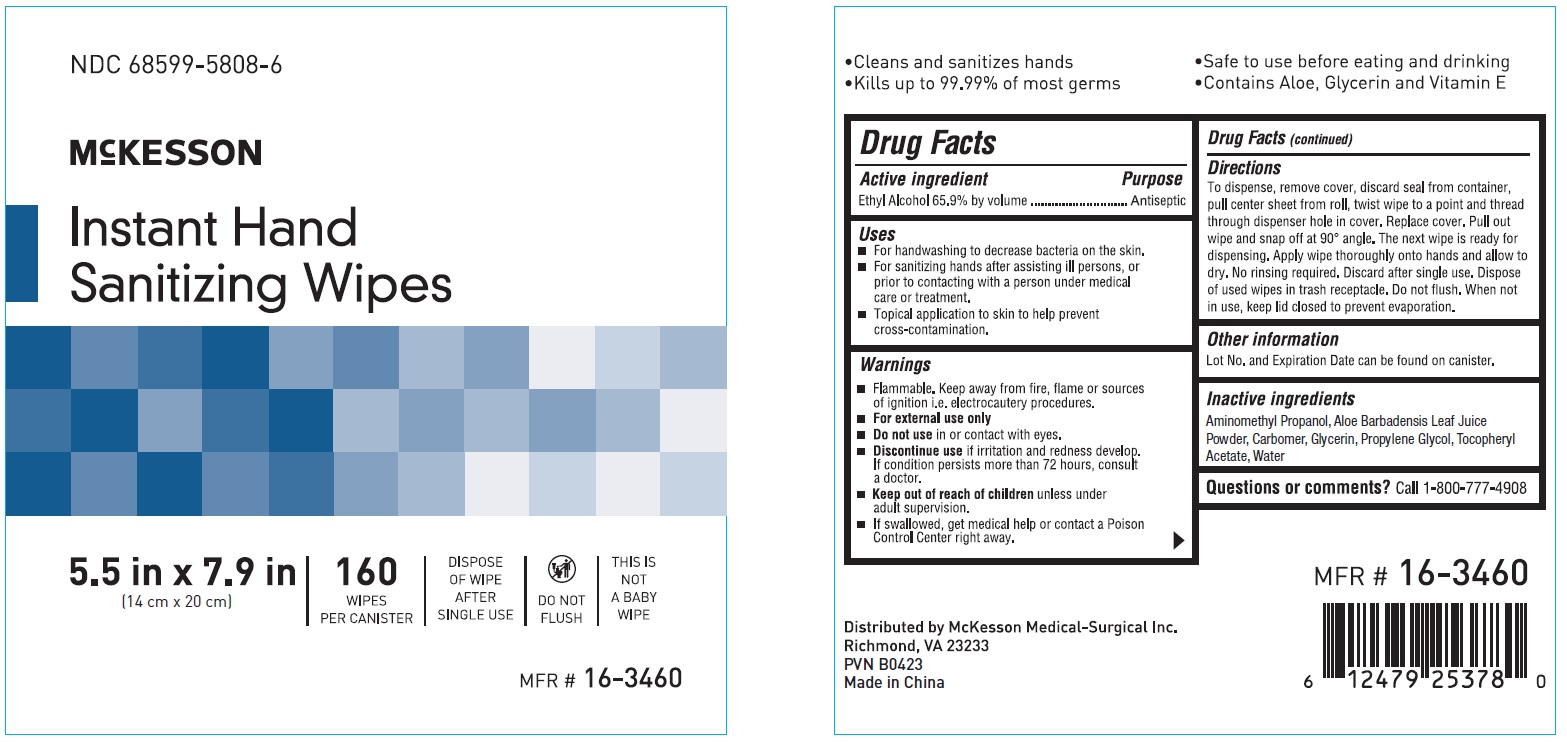
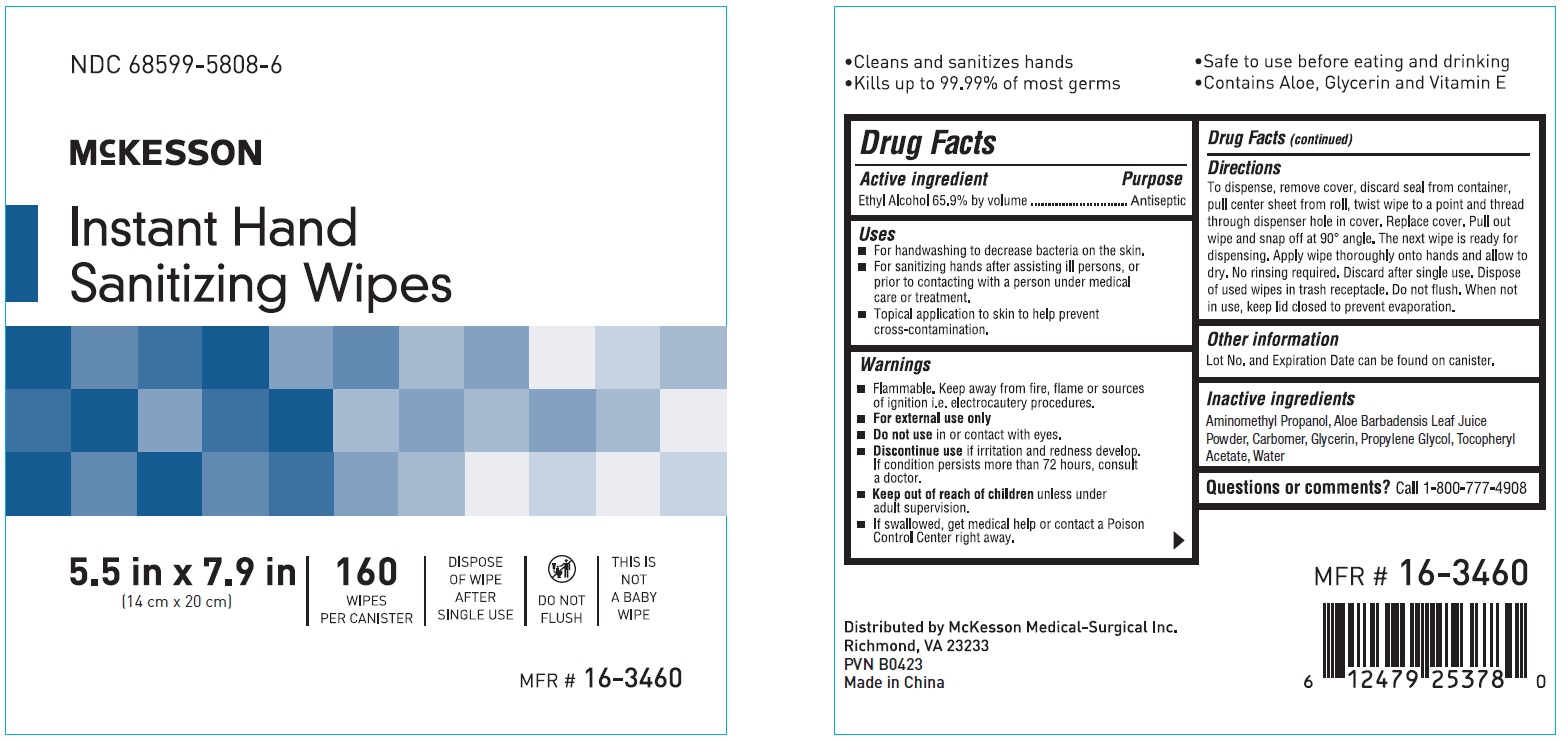
Label: INSTANT HAND SANITIZING WIPE- ethyl alcohol swab
- NDC Code(s): 68599-5808-6
- Packager: McKesson
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
To dispense, remove cover, discard seal from container, pull center sheet from roll, twist wipe to a point and thread through dispenser hole in cover.
Replace cover. Pull out wipe and snap off at 90 degree angle. The next wipe is ready for dispensing. Apply wipe thoroughly onto hands and allow to dry.
No rinsing required. Discard after single use. Dispose of used wipes in trash receptacle. Do not flush. When not in use, keep lid closed to prevent evaporation. - OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
INSTANT HAND SANITIZING WIPE
ethyl alcohol swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68599-5808 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 65.9 mg Inactive Ingredients Ingredient Name Strength AMINOMETHYLPROPANOL (UNII: LU49E6626Q) ALOE (UNII: V5VD430YW9) GLYCERIN (UNII: PDC6A3C0OX) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68599-5808-6 160 in 1 BOX; Type 0: Not a Combination Product 02/24/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 02/24/2020 Labeler - McKesson (023904428) Establishment Name Address ID/FEI Business Operations AHC LTD 413138557 manufacture(68599-5808)