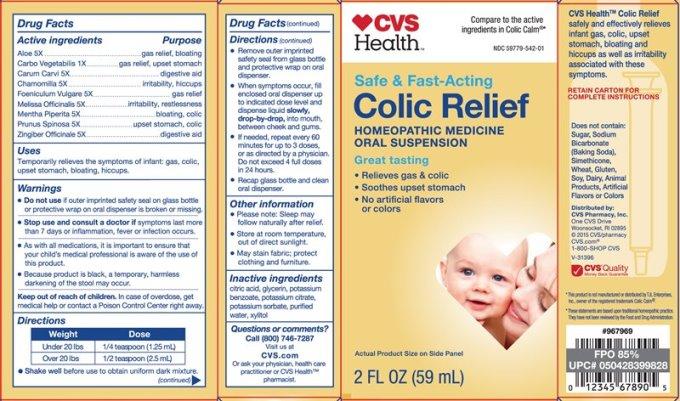
Label: COLIC RELIEF- aloe, carbo vegetabilis, carum carvi, chamomilla, foeniculum vulgare, melissa officinale, mentha piperita, prunus spinosa, zingiber officinale liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 59779-542-01 - Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 11, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS:
- USES:
-
WARNINGS:
• DO NOT USE IF OUTER IMPRINTED SAFETY SEAL ON GLASS BOTTLE OR PROTECTIVE WRAP ON ORAL DISPENSER ARE BROKEN OR MISSING.
• Stop use and consult a doctor if symptoms last more than 7 days or inflammation, fever or infection occurs.
• As with all medications, it is important to ensure that your child's medical professional is aware of the use of this product.
• Because product is black, a temporarily, harmless, darkening of the stool may occur.
• May stain fabric; protect clothing and furniture.
- KEEP OUT OF REACH OF CHILDREN:
-
DIRECTIONS:
• SHAKE WELL before use to obtain uniform dark mixture.
• Remove outer imprinted safety seal from glass bottle and protective wrap on oral dispenser.
• When symptoms occur, fill enclosed oral dispenser up to indicated dose level and dispense liquid slowly, drop-by-drop into mouth, between cheek and gums.
• If needed, repeat every 30 minutes for up to 3 doses, or as directed by a physician. Do not exceed 4 full doses in 24 hours.
• Recap glass bottle and clean oral dispenser.Weight Dose
Under 20 lbs. 1/4 teaspoon (1.25 mL)
Over 20 lbs. 1/2 teaspoon (2.5 mL) - OTHER SAFETY INFORMATION:
- INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
COLIC RELIEF
aloe, carbo vegetabilis, carum carvi, chamomilla, foeniculum vulgare, melissa officinale, mentha piperita, prunus spinosa, zingiber officinale liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-542 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALOE (UNII: V5VD430YW9) (ALOE - UNII:V5VD430YW9) ALOE 5 [hp_X] in 1 mL ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 1 [hp_X] in 1 mL CARAWAY SEED (UNII: W2FH8O2BBE) (CARAWAY SEED - UNII:W2FH8O2BBE) CARAWAY SEED 5 [hp_X] in 1 mL MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 5 [hp_X] in 1 mL FENNEL SEED (UNII: G3QC02NIE6) (FENNEL SEED - UNII:G3QC02NIE6) FENNEL SEED 5 [hp_X] in 1 mL MELISSA OFFICINALIS (UNII: YF70189L0N) (MELISSA OFFICINALIS - UNII:YF70189L0N) MELISSA OFFICINALIS 5 [hp_X] in 1 mL MENTHA PIPERITA (UNII: 79M2M2UDA9) (MENTHA PIPERITA - UNII:79M2M2UDA9) MENTHA PIPERITA 5 [hp_X] in 1 mL PRUNUS SPINOSA FLOWER BUD (UNII: 53Y84VPS2W) (PRUNUS SPINOSA FLOWER BUD - UNII:53Y84VPS2W) PRUNUS SPINOSA FLOWER BUD 5 [hp_X] in 1 mL GINGER (UNII: C5529G5JPQ) (GINGER - UNII:C5529G5JPQ) GINGER 5 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM BENZOATE (UNII: 763YQN2K7K) POTASSIUM CITRATE (UNII: EE90ONI6FF) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) XYLITOL (UNII: VCQ006KQ1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-542-01 1 in 1 CARTON 1 59 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/10/2015 Labeler - CVS Pharmacy (062312574) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(59779-542) , api manufacture(59779-542) , label(59779-542) , pack(59779-542)