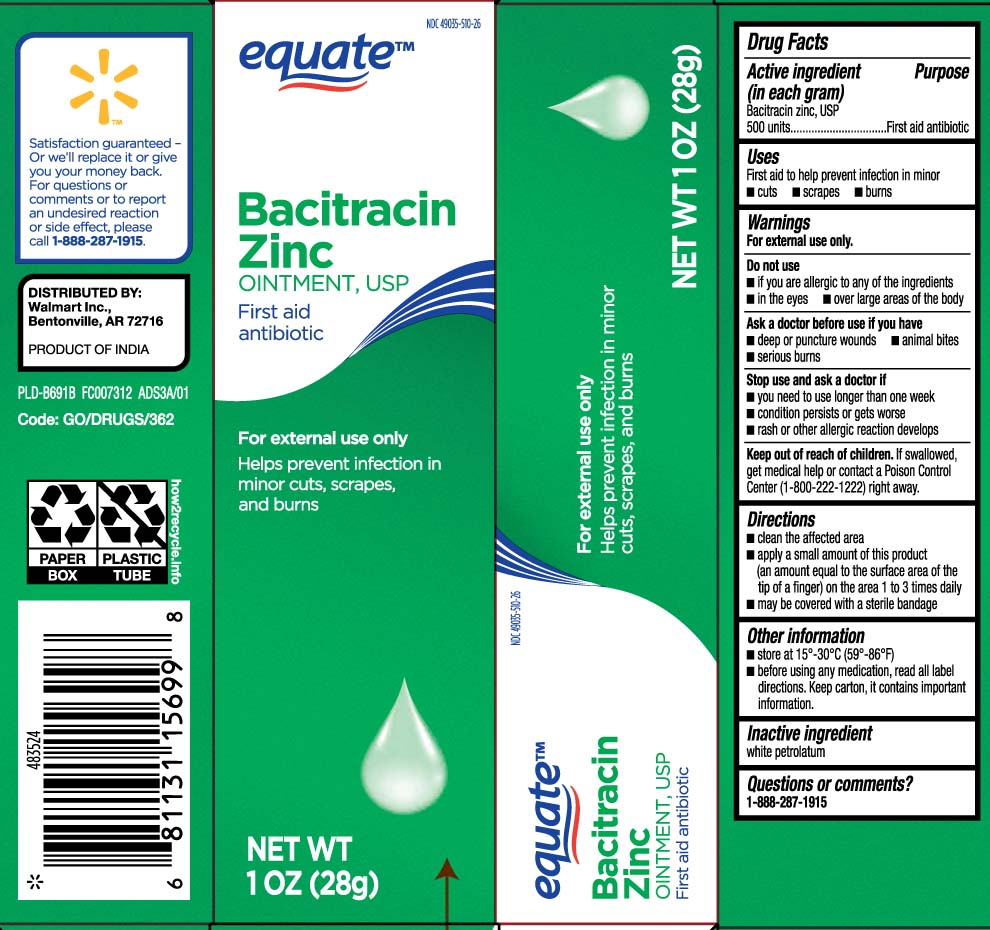
Label: BACITRACIN ZINC ointment
- NDC Code(s): 49035-510-26
- Packager: EQUATE (Wal-Mart Stores, Inc.) (see also WAL-MART INC)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each gram)
- Purpose
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Principal display panel
- Package label
-
INGREDIENTS AND APPEARANCE
BACITRACIN ZINC
bacitracin zinc ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49035-510 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 500 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49035-510-26 1 in 1 CARTON 08/07/2017 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 08/07/2017 Labeler - EQUATE (Wal-Mart Stores, Inc.) (see also WAL-MART INC) (051957769)