Label: AMINO ACID ORAL- amino acid solution
- NDC Code(s): 57319-528-04
- Packager: Clipper Distributing Company, LLC
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE
-
DOSAGE AND ADMINISTRATION
For use in drinking water. Supply fresh water daily.
Cattle: Administer 1 oz. per 10 pounds body weight in the drinking water consumed in one day.
Horses: Administer 10 ozs. per 100 pounds body weight in the drinking water consumed in one day.
Sheep and Swine: Administer 1 oz. per 10 pounds body weight i the drinking water consume in one day.
- STORAGE AND HANDLING
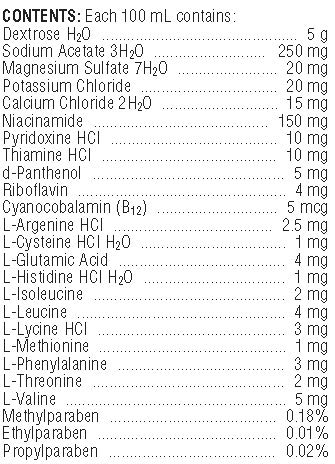
- COMPONENTS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AMINO ACID ORAL
amino acid solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:57319-528 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 5000 mg in 100 mL CALCIUM CHLORIDE ANHYDROUS (UNII: OFM21057LP) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 15 mg in 100 mL MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM SULFATE HEPTAHYDRATE 20 mg in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152, CHLORIDE ION - UNII:Q32ZN48698) POTASSIUM CHLORIDE 20 mg in 100 mL SODIUM ACETATE ANHYDROUS (UNII: NVG71ZZ7P0) (ACETATE ION - UNII:569DQM74SC, SODIUM CATION - UNII:LYR4M0NH37) SODIUM ACETATE ANHYDROUS 250 mg in 100 mL Inactive Ingredients Ingredient Name Strength VALINE (UNII: HG18B9YRS7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57319-528-04 500 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/01/2010 Labeler - Clipper Distributing Company, LLC (150711039) Registrant - Sparhawk Laboratories, Inc. (147979082) Establishment Name Address ID/FEI Business Operations Sparhawk Laboratories, Inc. 147979082 analysis, api manufacture, manufacture