Label: ALLERGY RELIEF- cetirizine hcl 10 mg tablet
- NDC Code(s): 50066-396-09, 50066-396-30, 50066-396-45, 50066-396-82
- Packager: Genomma Labs USA, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 20, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
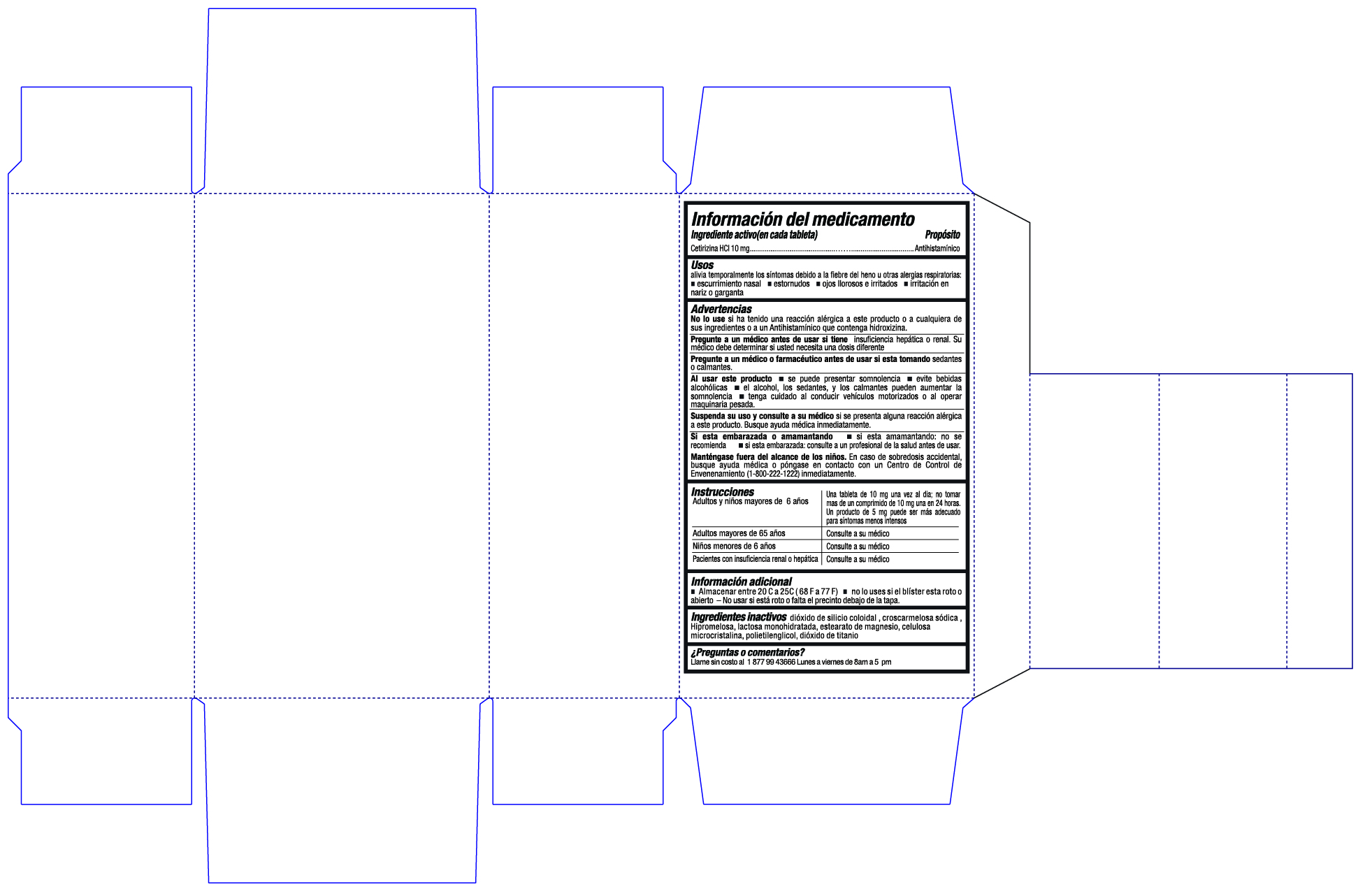
- Active ingredient
- Purpose
- Uses
-
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine
Ask doctor if you have
- liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when drivinga motor vehicle or operating machinery
- Keep Out of Reach of Children
-
Directions
- Adults and children 6 years and over: one 10mg tablet once daily; do not take more than one 10mg tablet in 24 hours. A 5mg product may be appropriate for less severe symptoms
- Adults 65 years and over: ask a doctor
- Children under 6 years of age: ask a doctor
- Consumers with liver or kidney disease: ask a doctor
- Other information
- Inactive Ingredients
- Questions or Comments
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
cetirizine hcl 10 mg tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50066-396 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) Product Characteristics Color white Score 2 pieces Shape RECTANGLE (pillow-shaped) Size 9mm Flavor Imprint Code G;4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50066-396-82 90 in 1 BOTTLE; Type 0: Not a Combination Product 11/06/2020 2 NDC:50066-396-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 11/06/2020 3 NDC:50066-396-45 3 in 1 CARTON 09/20/2019 10/26/2022 3 15 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:50066-396-09 1 in 1 CARTON 09/20/2019 10/26/2022 4 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209274 12/22/2017 Labeler - Genomma Labs USA, Inc (832323534)