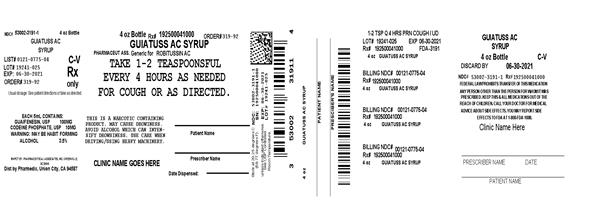
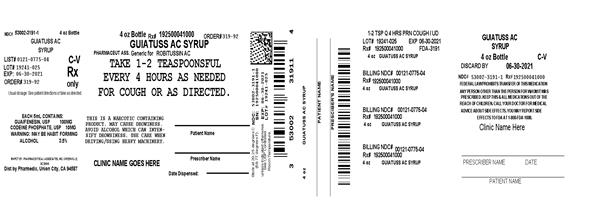
Label: GUAIFENESIN AND CODEINE PHOSPHATE solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 53002-3191-1 - Packager: RPK Pharmaceuticals, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0121-0775
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: CV
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 23, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Do not use
- in adults and children who have a chronic pulmonary disease or shortness of breath, or children who are taking other drugs, unless directed by a doctor.
Ask a doctor before use if you have
- a cough with too much phlegm (mucus)
- a persistent or chronic cough as occurs with smoking, asthma, chronic bronchitis, or emphysema
Ask a doctor or pharmacist before use if you are taking sedatives, tranquilizers and drugs used for depression, especially monoamine oxidase inhibitors (MAOIs). These combinations may cause greater sedation (drowsiness) than is caused by the product used alone.
-
Directions
- take every 4 hours
- do not exceed 6 doses in 24 hours
- a special measuring device should be used to give an accurate dose of this product to children under 6 years of age
- giving a higher dose than recommended by a doctor can result in serious side effects for a child
adults and children 12 years and over 10 mL (2 teaspoonfuls) children 6 to under 12 years of age 5 mL (1 teaspoonful) children under 6 years of age Consult a doctor - Other information
- Inactive ingredients
- Questions or comments?
- HOW SUPPLIED
- Guiatuss AC Syrup C-V
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN AND CODEINE PHOSPHATE
guaifenesin and codeine phosphate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53002-3191(NDC:0121-0775) Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL CODEINE PHOSPHATE (UNII: GSL05Y1MN6) (CODEINE ANHYDROUS - UNII:UX6OWY2V7J) CODEINE PHOSPHATE 10 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GLYCERIN (UNII: PDC6A3C0OX) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL (UNII: 506T60A25R) Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53002-3191-1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 10/01/2006 Labeler - RPK Pharmaceuticals, Inc. (147096275) Establishment Name Address ID/FEI Business Operations RPK Pharmaceuticals, Inc. 147096275 RELABEL(53002-3191)