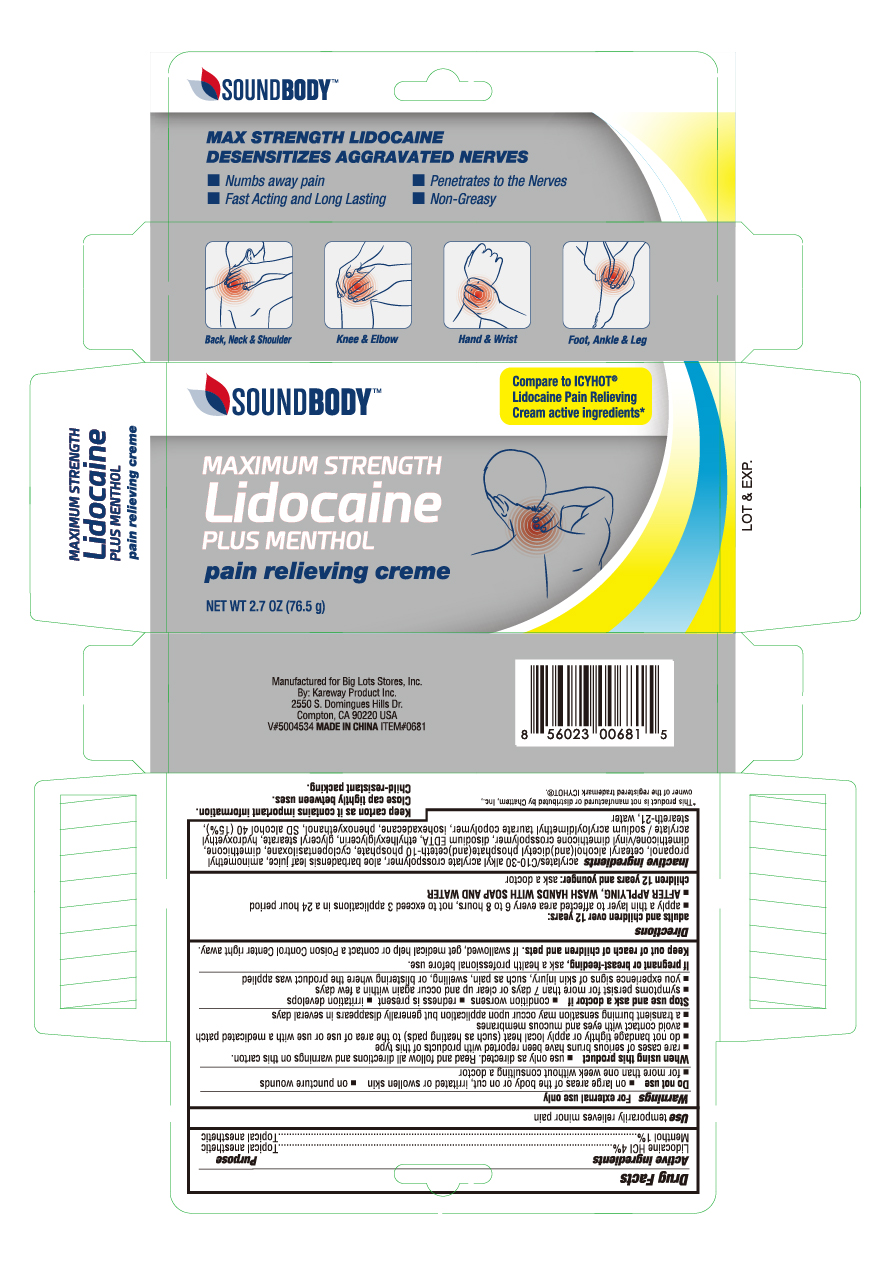
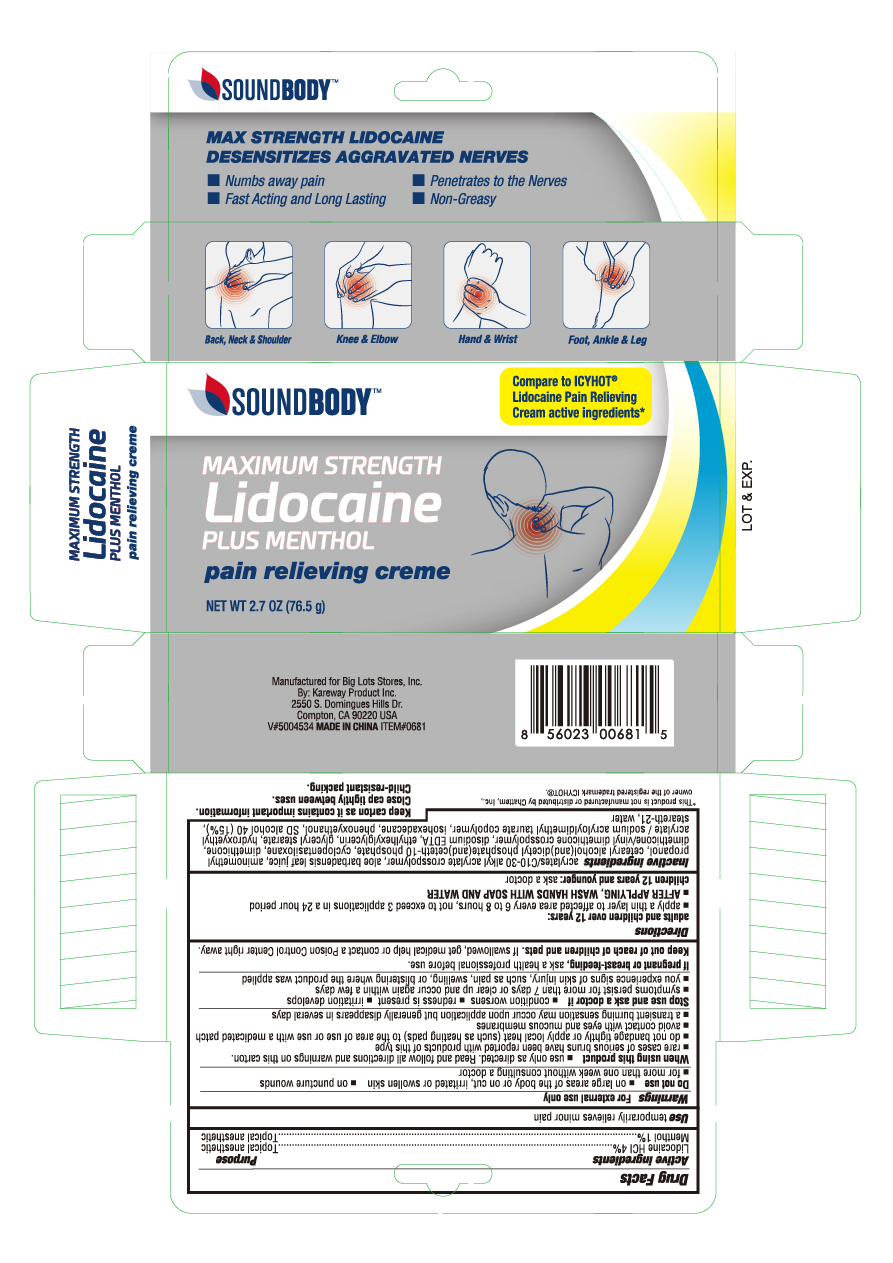
Label: LIDOCAINE WITH MENTHOL- lidocaine hcl and menthol cream
- NDC Code(s): 67510-0681-2
- Packager: Kareway Product, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 19, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purposes
- Active Ingredients
- Purposes
- Uses
-
Warnings
For external use only
Do not use
- on large areas of the body or on cut, irritated or swollen skin
- on puncture wounds
- for more than one week without consulting a doctor
When using this product
- Use only as directed. Read and follow all directions and warnings on this label
- rare cases of serious burns have been reported with products of this type
- do not bandage tightly or apply local heat (such as heating pads) to the area of use or use with a medicated patch
- avoid contact with eyes and mucous membranes
- a transient burning sensation may occur upon application but generally disappears in several days
- on large areas of the body or on cut, irritated or swollen skin
- Directions
-
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, aloe barbadensis leaf juice, aminomethyl propanol, cetearyl alcohol(and)dicetyl phosphate(and)ceteth-10 phosphate, cyclopentasiloxane, dimethicone, dimethicone/vinyl dimethicone crosspolymer, disodium EDTA, ethylhexylglycerin, glyceryl stearate, hydroxyethyl acrylate / sodium acryloyldimethyl taurate copolymer, isohexadecane, phenoxyethanol, SD alcohol 40 (15%), steareth-21, water
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIDOCAINE WITH MENTHOL
lidocaine hcl and menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67510-0681 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 0.4 g in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (55000 CPS) (UNII: 59TL3WG5CO) ALOE VERA LEAF (UNII: ZY81Z83H0X) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) DIHEXADECYL PHOSPHATE (UNII: 2V6E5WN99N) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ISOHEXADECANE (UNII: 918X1OUF1E) POLYSORBATE 60 (UNII: CAL22UVI4M) ALCOHOL (UNII: 3K9958V90M) STEARETH-21 (UNII: 53J3F32P58) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67510-0681-2 1 in 1 CARTON 02/17/2020 1 76.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 02/17/2020 Labeler - Kareway Product, Inc. (121840057)