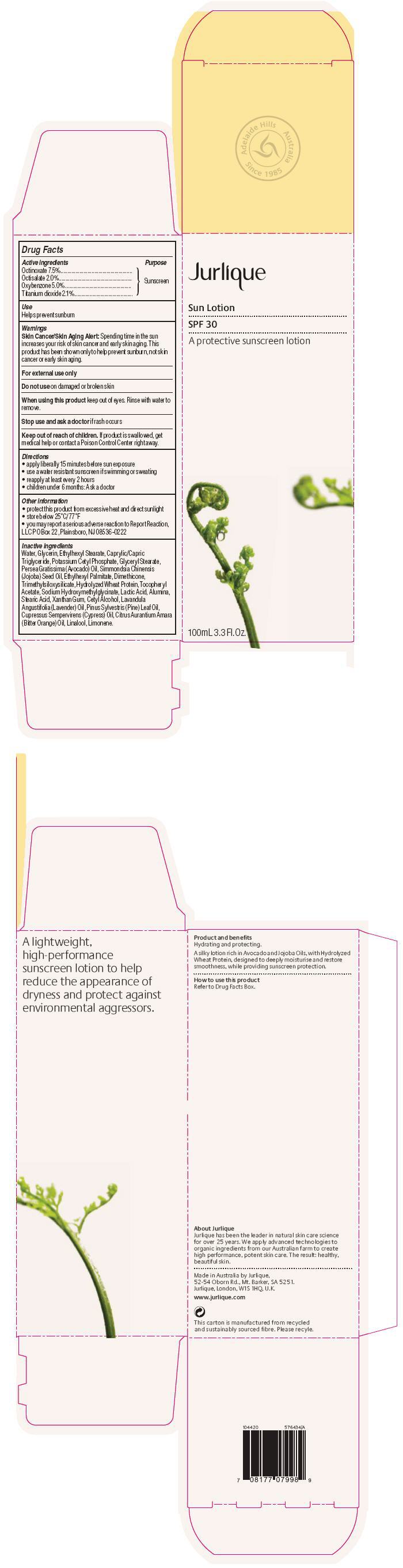
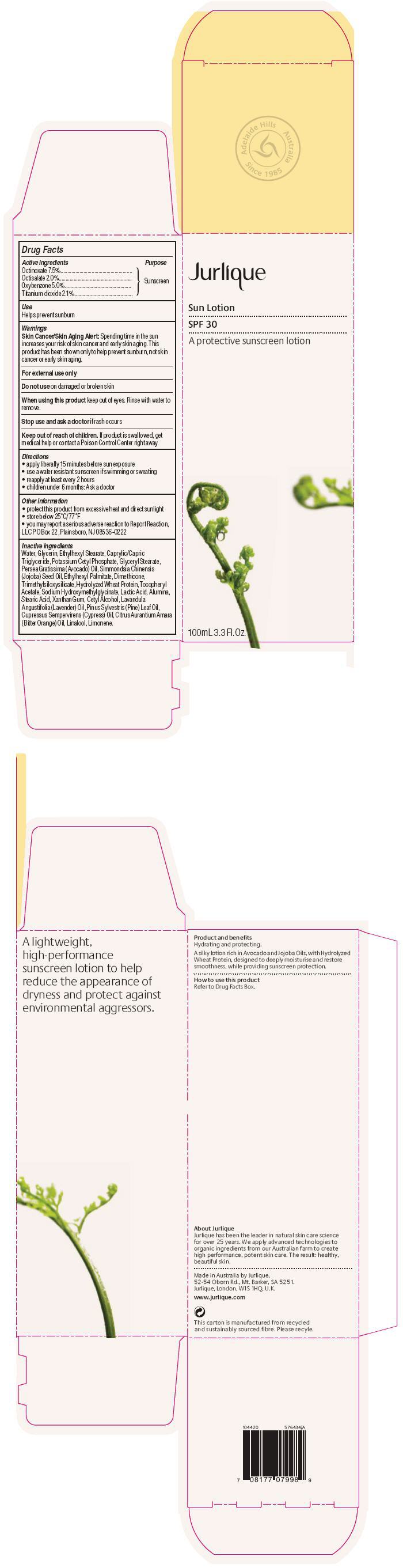
Label: JURLIQUE SUN SUNSCREEN SPF 30- octinoxate, octisalate, oxybenzone, and titanium dioxide cream, augmented
-
Contains inactivated NDC Code(s)
NDC Code(s): 68105-005-01, 68105-005-02, 68105-005-03, 68105-005-04 - Packager: Jurlique International Pty. Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 21, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water, Glycerin, Ethylhexyl Stearate, Caprylic/Capric Triglyceride, Potassium Cetyl Phosphate, Glyceryl Stearate, Persea Gratissima (Avocado) Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Ethylhexyl Palmitate, Dimethicone, Trimethylsiloxysilicate, Hydrolyzed Wheat Protein, Tocopheryl Acetate, Sodium Hydroxymethylglycinate, Lactic Acid, Alumina, Stearic Acid, Xanthan Gum, Cetyl Alcohol, Lavandula Angustifolia (Lavender) Oil, Pinus Sylvestris (Pine) Leaf Oil, Cupressus Sempervirens (Cypress) Oil, Citrus Aurantium Amara (Bitter Orange) Oil, Linalool, Limonene.
- PRINCIPAL DISPLAY PANEL - 100mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
JURLIQUE SUN SUNSCREEN SPF 30
octinoxate, octisalate, oxybenzone, and titanium dioxide cream, augmentedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68105-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 75 mg in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 20 mg in 1 mL Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 50 mg in 1 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 21 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Ethylhexyl Stearate (UNII: EG3PA2K3K5) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Potassium Cetyl Phosphate (UNII: 03KCY6P7UT) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) AVOCADO OIL (UNII: 6VNO72PFC1) JOJOBA OIL (UNII: 724GKU717M) Ethylhexyl Palmitate (UNII: 2865993309) Dimethicone (UNII: 92RU3N3Y1O) HYDROLYZED WHEAT PROTEIN (ENZYMATIC, 3000 MW) (UNII: J2S07SB0YL) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Sodium Hydroxymethylglycinate (UNII: DIG6BWZ9XT) Lactic Acid (UNII: 33X04XA5AT) Aluminum Oxide (UNII: LMI26O6933) Stearic Acid (UNII: 4ELV7Z65AP) Xanthan Gum (UNII: TTV12P4NEE) Cetyl Alcohol (UNII: 936JST6JCN) LAVENDER OIL (UNII: ZBP1YXW0H8) PINE NEEDLE OIL (PINUS SYLVESTRIS) (UNII: 5EXL5H740Y) BITTER ORANGE OIL (UNII: 9TLV70SV6I) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+/-)- (UNII: 9MC3I34447) Product Characteristics Color WHITE (Off-White) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68105-005-03 1 in 1 CARTON 1 NDC:68105-005-01 100 mL in 1 BOTTLE, PUMP 2 NDC:68105-005-04 1 in 1 CARTON 2 NDC:68105-005-02 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/01/2013 Labeler - Jurlique International Pty. Ltd. (752025791) Establishment Name Address ID/FEI Business Operations Jurlique International Pty. Ltd. 752025791 MANUFACTURE(68105-005)