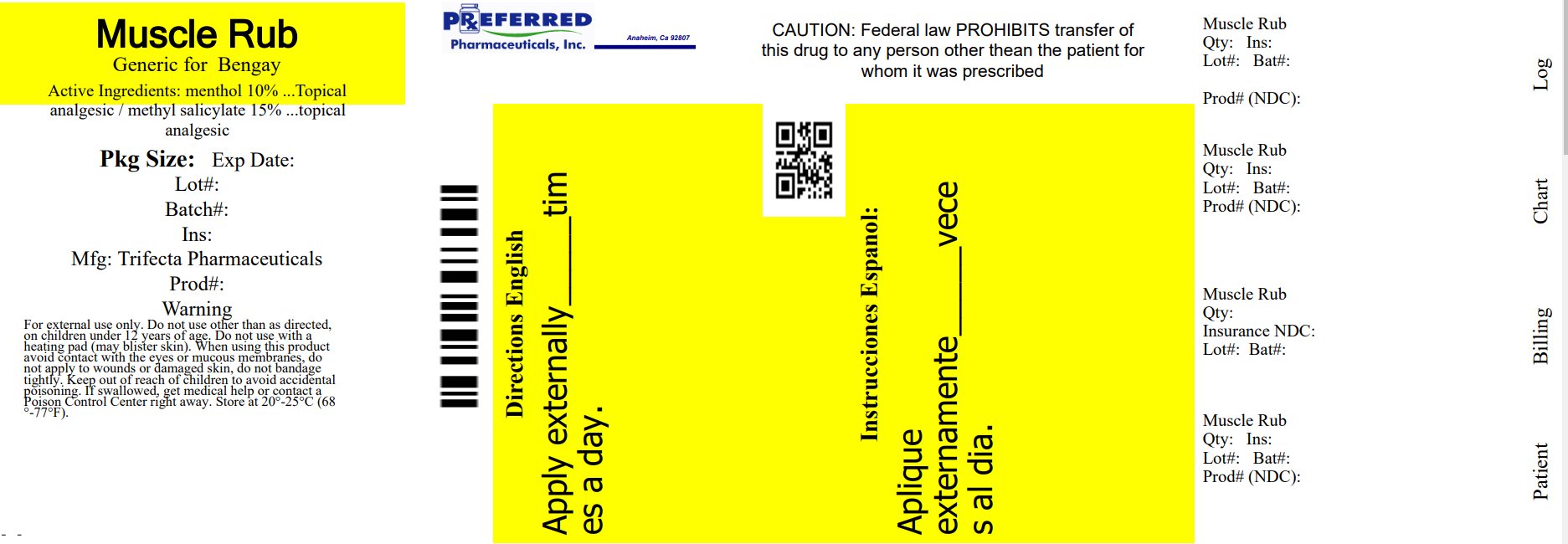
Label: MUSCLE RUB CREAM- menthol 10%, methyl salicylate 15% cream
- NDC Code(s): 68788-8544-8
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 69396-110
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Active Ingredient
- PURPOSE
- INDICATIONS & USAGE
- Warnings
- Stop Use and ask a doctor
- Keep out of the reach of Children
- Directions
- Other Information
- Inactive Ingredients
-
Distributed By
Trifecta Pharmaceuticals USA, LLC.
101 NE Third Avenue, Suite 1500
Ft. Lauderdale, FL. 33301
www.trifecta-pharma.com
Made in China
This product is not manufactured or distributed by Johnson & Johnson Consumer Products Company, a division of Johnson & Johnson consumer companies Inc. owner of the registered trademark BENGAY®.
Relabeled By: Preferred Pharmaceuticals Inc.
NDC 68788-8544-8 - Packaging
-
INGREDIENTS AND APPEARANCE
MUSCLE RUB CREAM
menthol 10%, methyl salicylate 15% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68788-8544(NDC:69396-110) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 10 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 15 g in 100 g Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) TROLAMINE (UNII: 9O3K93S3TK) PROPYLPARABEN (UNII: Z8IX2SC1OH) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68788-8544-8 1 in 1 BOX 11/06/2023 1 85 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 348 11/06/2023 Labeler - Preferred Pharmaceuticals Inc. (791119022) Registrant - Preferred Pharmaceuticals Inc. (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals Inc. 791119022 RELABEL(68788-8544)