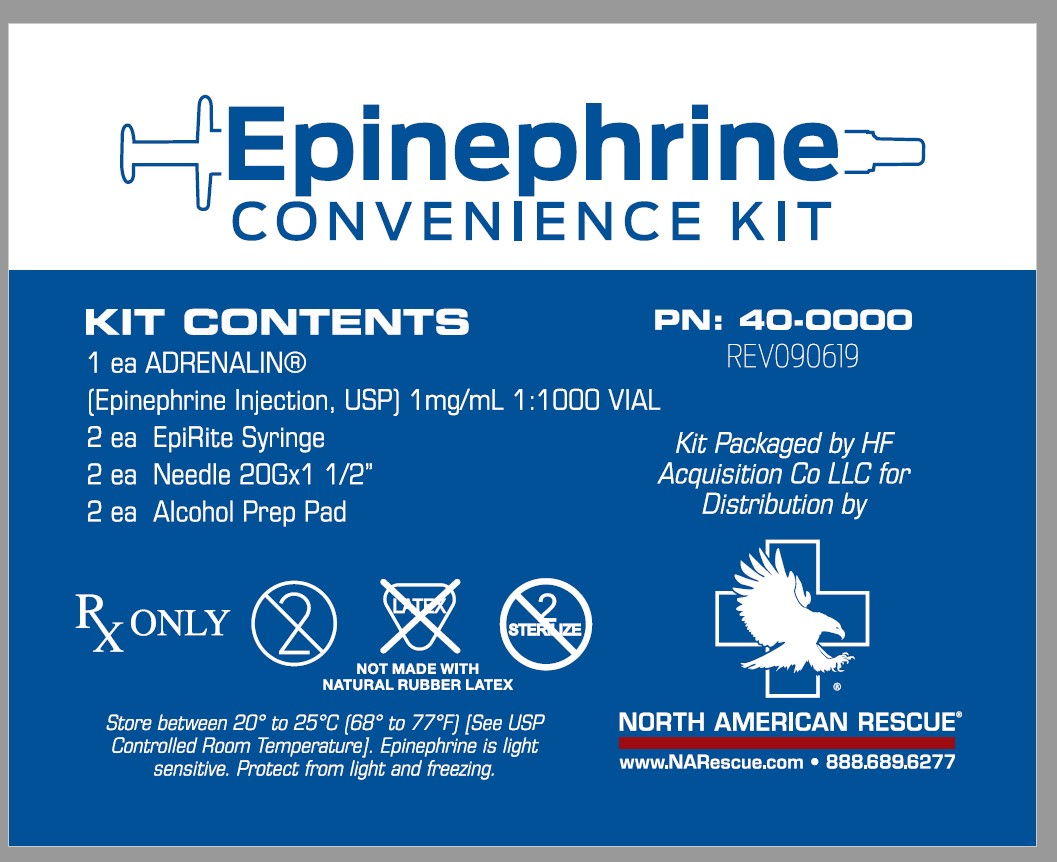
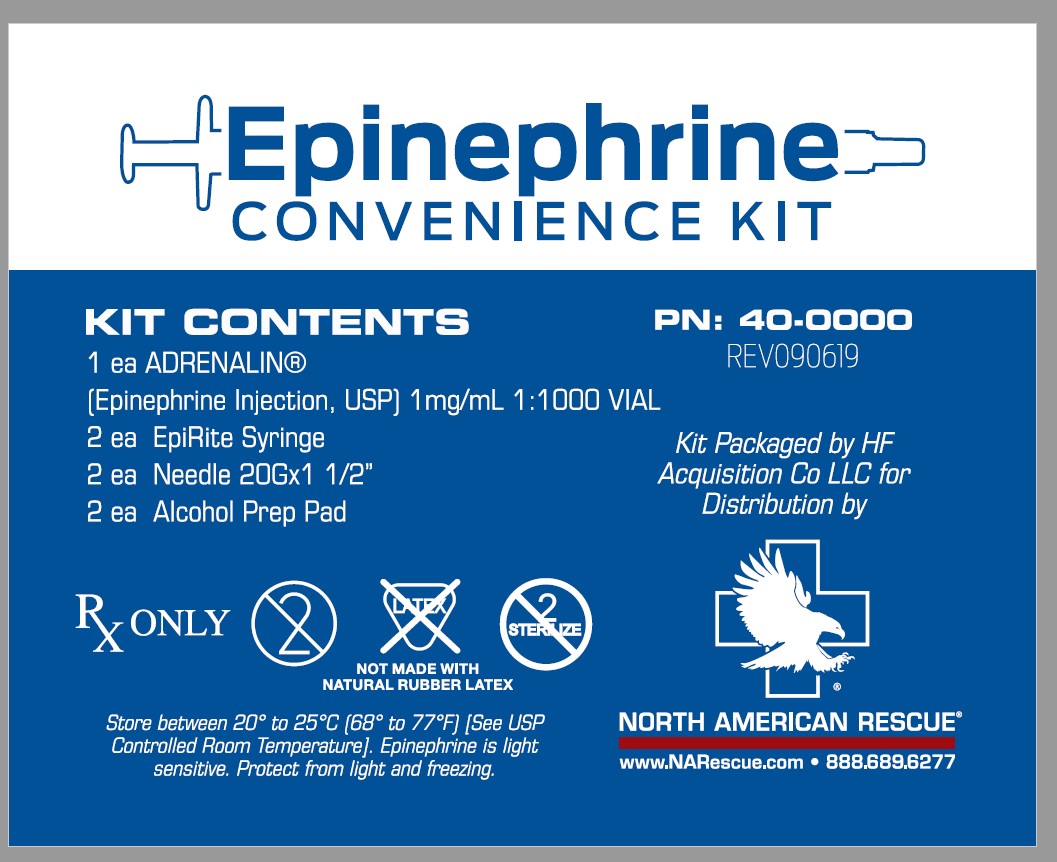
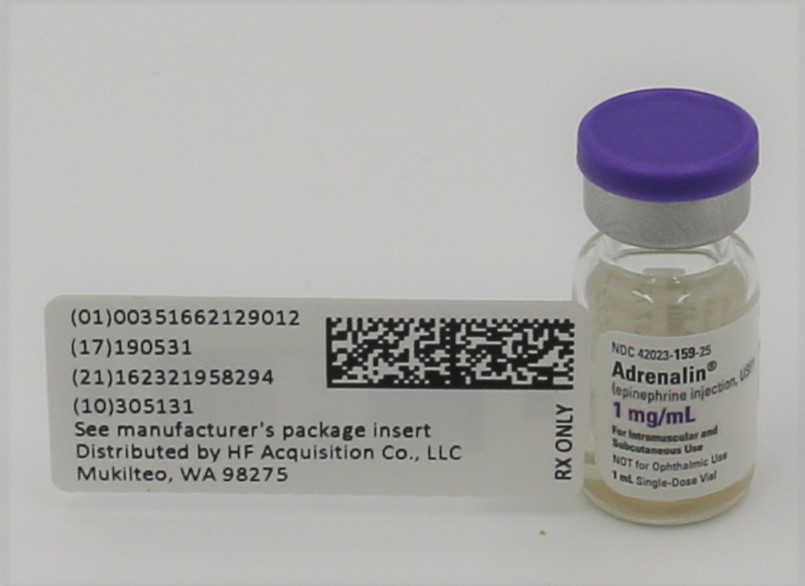
Label: EPINEPHRINE CONVENIENCE KIT- epinephrine kit
- NDC Code(s): 51662-1290-1, 51662-1507-1
- Packager: HF Acquisition Co LLC, DBA HealthFirst
- This is a repackaged label.
- Source NDC Code(s): 42023-159
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated March 6, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ADRENALIN safely and effectively. See full prescribing information for ADRENALIN.
ADRENALIN (epinephrine injection) 1 mg/mL, for
intramuscular, subcutaneous, and intravenous use
Initial U.S. Approval: 1939RECENT MAJOR CHANGES
Indications and Usage (1.2) 01/2019
Dosage and Administration (2.3) 01/2019
Warnings and Precautions (5.3, 5.4, 5.5, 5.6, 5.7) 01/2019
INDICATIONS AND USAGE
Adrenalin® is a non-selective alpha and beta adrenergic agonist indicated for:
Emergency treatment of allergic reactions (Type 1), including anaphylaxis ( 1-1.1)
To increase mean arterial blood pressure in adult patients with hypotension associated with septic shock ( 1-1.2)DOSAGE AND ADMINISTRATION
Anaphylaxis:
Adults and Children 30 kg (66 lbs) or more: 0.3 mg to 0.5 mg (0.3 mL to 0.5 mL) intramuscularly or subcutaneously into anterolateral aspect of the thigh every 5 to 10 minutes as necessary ( 2-2.2)
Children 30 kg (66 lbs) or less: 0.01 mg/kg (0.01 mL/kg), up to 0.3 mg (0.3 mL), intramuscularly or subcutaneously into anterolateral aspect of the thigh every 5 to 10 minutes as necessary ( 2-2.2)Hypotension associated with septic shock:
Dilute epinephrine in dextrose solution prior to infusion ( 2-2.3)
Infuse epinephrine into a large vein ( 2-2.3)
Intravenous infusion rate of 0.05 mcg/kg/min to 2 mcg/kg/min, titrated to achieve desired mean arterial pressure ( 2-2.3)
Wean gradually ( 2-2.3)
See Full Prescribing Information for instructions on dilution and administration of the injection.DOSAGE FORMS AND STRENGTHS
Injection: 1 mg/mL single dose vial and 30 mg/30 mL (1 mg/mL) multiple dose vial ( 3)
CONTRAINDICATIONS
None ( 4)
WARNINGS AND PRECAUTIONS
Do not inject into buttocks, digits, hands, or feet ( 5-5.1)
Avoid extravasation into tissues, which can cause local necrosis ( 5-5.3)
May aggravate angina pectoris or produce ventricular arrhythmias ( 5-5.7)ADVERSE REACTIONS
Common adverse reactions to systemically administered epinephrine include anxiety, tremor, weakness, dizziness, sweating, palpitations and pallor ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Par Pharmaceutical at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Drugs that counter the pressor effects of epinephrine include alpha blockers, vasodilators such as nitrates, diuretics, antihypertensives and ergot alkaloids. ( 7-7.1)
Drugs that potentiate the effects of epinephrine include sympathomimetics, beta blockers, tricyclic antidepressants, MAO inhibitors, COMT inhibitors, clonidine, doxapram, oxytocin, levothyroxine sodium, quinidine and certain antihistamines. ( 7-7.2)
Drugs that increase the arrhythmogenic potential of epinephrine include beta blockers, cyclopropane and halogenated hydrocarbon anesthetics, antihistamines, exogenous thyroid hormones, diuretics, and cardiac glycosides. Observe for development of cardiac arrhythmias. ( 7-7.3)
Potassium-depleting drugs, including corticosteroids, diuretics, and theophylline, potentiate the hypokalemic effects of epinephrine. ( 7-7.4)USE IN SPECIFIC POPULATIONS
Elderly patients and pregnant women may be at greater risk of developing adverse reactions when epinephrine is administered parenterally ( 8-8.1, 8-8.5)
Pregnancy: May cause fetal harm ( 8-8.1)See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2019
- KIT CONTENTS
-
FULL PRESCRIBING INFORMATION: CONTENTS*
1INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Incorrect Locations of Injection
5.2 Serious Infections at the Injection Site OK
5.3 Disease Interactions
5.4 Allergic Reactions Associated with Sulfite
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION*
Sections or subsections omitted from the full prescribing information are not listed. -
1 INDICATIONS & USAGE
Adrenalin® is available as a single-use 1 mL vial and a multiple-use 30 mL vial for intramuscular and subcutaneous use.
Emergency treatment of allergic reactions (Type I), including anaphylaxis, which may result from allergic reactions to insect stings, biting insects, foods, drugs, sera, diagnostic testing substances and other allergens, as well as idiopathic anaphylaxis or exercise-induced anaphylaxis. The signs and symptoms associated with anaphylaxis include flushing, apprehension, syncope, tachycardia, thready or unobtainable pulse associated with hypotension, convulsions, vomiting, diarrhea and abdominal cramps, involuntary voiding, airway swelling, laryngospasm, bronchospasm, pruritus, urticaria or angioedema, swelling of the eyelids, lips, and tongue.
-
2 DOSAGE & ADMINISTRATIN
Inject Adrenalin® intramuscularly or subcutaneously into the anterolateral aspect of the thigh, through clothing if necessary. When administering to a child, to minimize the risk of injection related injury, hold the leg firmly in place and limit movement prior to and during an injection. The injection may be repeated every 5 to 10 minutes as necessary. For intramuscular administration, use a needle long enough (at least 1/2 inch to 5/8 inch) to ensure the injection is administered into the muscle. Monitor the patient clinically for the severity of the allergic reaction and potential cardiac effects of the drug, with repeat doses titrated to effect. Do not administer repeated injections at the same site, as the resulting vasoconstriction may cause tissue necrosis.
Inspect visually for particulate matter and discoloration prior to administration. Do not use if the solution is colored or cloudy, or if it contains particulate matter.
Adults and Children 30 kg (66 lbs) or more: 0.3 to 0.5 mg (0.3 mL to 0.5 mL) of undiluted Adrenalin® administered intramuscularly or subcutaneously in the anterolateral aspect of the thigh, up to a maximum of 0.5 mg (0.5 mL) per injection, repeated every 5 to 10 minutes as necessary. Monitor clinically for reaction severity and cardiac effects.
Children less than 30 kg (66 lbs): 0.01 mg/kg (0.01 mL/kg) of undiluted Adrenalin® administered intramuscularly or subcutaneously in the anterolateral aspect of the thigh, up to a maximum of 0.3 mg (0.3 mL) per injection, repeated every 5 to 10 minutes as necessary. Monitor clinically for reaction severity and cardiac effects.
- 3 DOSAGE FORMS & STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Incorrect Locations of Injection
Injection into the anterolateral aspect of the thigh (vastus lateralis muscle) is the most appropriate location for administration because of its location, size, and available blood flow. Injection into (or near) smaller muscles, such as in the deltoid, is not recommended due to possible differences in absorption associated with this use.
Do not administer repeated injections of epinephrine at the same site, as the resulting vasoconstriction may cause tissue necrosis.
Do not inject into buttock. Injection into the buttock may not provide effective treatment of anaphylaxis and has been associated with the development of Clostridial infections (gas gangrene). Cleansing with alcohol does not kill bacterial spores, and therefore, does not lower this risk.
Do not inject into digits, hands, or feet. Epinephrine is a strong vasoconstrictor. Accidental injection into the digits, hands or feet may result in loss of blood flow to the affected area and has been associated with tissue necrosis.
5.2 Serious Infections at the Injection Site
Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported at the injection site following epinephrine injection for anaphylaxis. Clostridium spores can be present on the skin and introduced into the deep tissue with subcutaneous or intramuscular injection. While cleansing with alcohol may reduce presence of bacteria on the skin, alcohol cleansing does not kill Clostridium spores. To decrease the risk of Clostridium infection, do not inject Adrenalin® into the buttock [see Warnings and Precautions (5.1)]. Advise patients to seek medical care if they develop signs or symptoms of infection, such as persistent redness, warmth, swelling, or tenderness, at the epinephrine injection site.
5.3 Disease Interactions
Some patients may be at greater risk for developing adverse reactions after systemic epinephrine administration. Despite these concerns, the presence of these conditions is not a contraindication to epinephrine administration in an acute, life-threatening situation.
Patients with Heart Disease
Epinephrine should be administered with caution in patients who have heart disease, including patients with cardiac arrhythmias, coronary artery or organic heart disease, cerebrovascular disease, or hypertension. In such patients, or in patients who are on drugs that may sensitize the heart to arrhythmias, epinephrine may precipitate or aggravate angina pectoris as well as produce ventricular arrhythmias [see Drug Interactions ( 7) and Adverse Reactions ( 6)].
Other Patients and Diseases
Epinephrine should be administered with caution to patients with hyperthyroidism, Parkinson's disease, diabetes mellitus, pheochromocytoma, elderly individuals, and pregnant women. Patients with Parkinson's disease may experience psychomotor agitation or notice a temporary worsening of symptoms. Diabetic patients may experience transient increases in blood sugar.
5.4 Allergic Reactions Associated with Sulfite
Adrenalin® contains sodium bisulfite which may cause mild to severe allergic reactions including anaphylaxis or asthmatic episodes in susceptible individuals. However, the presence of bisulfite in this product should not preclude its use for the treatment of serious allergic or other emergency situations even if the patient is sulfite-sensitive, as the alternatives to using epinephrine in a life-threatening situation may not be satisfactory.
-
6 ADVERSE REACTIONS
Common adverse reactions to systemically administered epinephrine include anxiety, apprehensiveness, restlessness, tremor, weakness, dizziness, sweating, palpitations, pallor, nausea and vomiting, headache, and respiratory difficulties. These symptoms occur in some persons receiving therapeutic doses of epinephrine, but are more likely to occur in patients with heart disease, hypertension, or hyperthyroidism [see Warnings and Precautions 5-(5.3)].
Due to the lack of randomized, controlled clinical trials of epinephrine for the treatment of anaphylaxis, the true incidence of adverse reactions associated with the systemic use of epinephrine is difficult to determine. Adverse reactions reported in observational trials, case reports, and studies are listed below by body system:
Cardiovascular: angina, arrhythmias, hypertension, pallor, palpitations, tachyarrhythmia, tachycardia, vasoconstriction, ventricular ectopy and stress cardiomyopathy.
Angina may occur in patients with coronary artery disease [see Warnings and Precautions 5-(5.3)].
Arrhythmias, including fatal ventricular fibrillation, have occurred, particularly in patients with underlying organic heart disease or patients receiving drugs that sensitize the heart to arrhythmias [see Warnings and Precautions 5-(5.3)].
Rapid rises in blood pressure associated with epinephrine use have produced cerebral hemorrhage, particularly in elderly patients with cardiovascular disease [see Warnings and Precautions 5-(5.3)].
Respiratory: respiratory difficulties.
Neurological: dizziness , disorientation , excitability , headache , impaired memory , lightheadedness , nervousness , panic, psychomotor agitation, sleepiness , tingling , tremor, and weakness.
Psychiatric: anxiety, apprehensiveness, restlessness.
Gastrointestinal: nausea, vomiting.
Other:
Patients with Parkinson's disease may experience psychomotor agitation or a temporary worsening of symptoms [see Warnings and Precautions 5-(5.3)].
Diabetic patients may experience transient increases in blood sugar [see Warnings and Precautions 5-(5.3)].
Accidental injection into the digits, hands or feet may result in loss of blood flow to the affected area [see Warnings and Precautions 5-(5.1)]. Adverse events experienced as a result of an injection into these areas include increased heart rate, local reactions including injection site pallor, coldness, hypoesthesia, and tissue loss, or injury at the injection site resulting in bruising, bleeding, discoloration, erythema, and skeletal injury.
Injection into the buttock has resulted in cases of gas gangrene [see Warnings and Precautions 5-(5.1)].
Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported following epinephrine injection in the thigh [see Warnings and Precautions 5-(5.2)].
Skin: sweating.
To report SUSPECTED ADVERSE REACTIONS, contact Par Pharmaceutical at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
7 DRUG INTERACTIONS
Epinephrine should be administered cautiously to patients taking other sympathomimetic agents because of the possibility of additive effects.
Patients who are concomitantly receiving cardiac glycosides, digitalis, diuretics, quinidine, and other antiarrhythmics should be observed carefully for the development of cardiac arrhythmias [see Warnings and Precautions 5-(5.3) and Adverse Reactions ( 6)].
Administer epinephrine cautiously to patients receiving halogenated hydrocarbon general anesthetics, such as halothane, as coadministration may result in arrhythmias.
The effects of epinephrine may be potentiated by tricyclic antidepressants such as imipramine, monoamine oxidase inhibitors (MAOI), levothyroxine sodium, and certain antihistamines, notably diphenhydramine, tripelannamine, and dexchlorpheniramine.
The cardiostimulating and bronchodilating effects of epinephrine are antagonized by beta-adrenergic blocking drugs, such as propranolol.
The vasoconstricting and hypertensive effects of epinephrine are antagonized by alpha-adrenergic blocking drugs, such as phentolamine.
Ergot alkaloids may reverse the pressor effects of epinephrine.
Epinephrine should not be used to counteract circulatory collapse or hypotension caused by phenothiazines, as a reversal of the pressor effects of epinephrine may result in further lowering of blood pressure.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category C.
There are no adequate and well-controlled studies in pregnant women. Epinephrine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus (fetal anoxia, spontaneous abortion, or both). Epinephrine is teratogenic in rabbits, mice and hamsters dosed during organogenesis.
Epinephrine has been shown to have teratogenic effects (including gastroschisis and embryonic lethality) when administered subcutaneous in rabbits at approximately 15 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m2 basis at a maternal subcutaneous dose of 1.2 mg/kg/day for two to three days).
In mice, teratogenic effects (including embryonic lethality) were observed at approximately 3 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m2 basis at maternal subcutaneous dose of 1 mg/kg/day for 10 days). These effects were not seen in mice at approximately 2 times the maximum recommended daily intramuscular or subcutaneous dose (on a mg/m2 basis at a subcutaneous maternal dose of 0.5 mg/kg/day for 10 days).
In hamsters, teratogenic effects were observed at approximately 2 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m2 basis at a maternal subcutaneous dose of 0.5 mg/kg/day for 4 days).
8.2 Labor and Delivery
Use with caution during labor and delivery. Although epinephrine improves maternal hypotension associated with anaphylaxis, it may result in uterine vasoconstriction, decreased uterine blood flow, and fetal anoxia.
8.3 Nursing Mothers
It is not known whether epinephrine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when epinephrine is administered to a nursing woman.
8.4 Pediatric Use
Clinical use data support weight-based dosing for treatment of anaphylaxis in pediatric patients, and other reported clinical experience with the use of epinephrine suggests that the adverse reactions seen in children are similar in nature and extent to those both expected and reported in adults.
8.5 Geriatric Use
Clinical studies for the treatment of anaphylaxis have not been performed in subjects aged 65 and over to determine whether they respond differently from younger subjects. However, other reported clinical experience with use of epinephrine for the treatment of anaphylaxis has identified that geriatric patients may be particularly sensitive to the effects of epinephrine. Therefore, for the treatment of anaphylaxis, consider starting with a lower dose to take into account potential concomitant disease or other drug therapy.
-
10 OVERDOSAGE
Overdosage of epinephrine may produce extremely elevated arterial pressure, which may result in cerebrovascular hemorrhage, particularly in elderly patients. Overdosage may also result in pulmonary edema because of peripheral vascular constriction together with cardiac stimulation. Treatment consists of a rapidly acting α-adrenergic blocking drug and respiratory support.
Epinephrine is rapidly inactivated in the body and treatment following overdose with epinephrine is primarily supportive. If necessary, pressor effects may be counteracted by rapidly acting vasodilators or α-adrenergic blocking drugs. If prolonged hypotension follows such measures, it may be necessary to administer another pressor drug.
Epinephrine overdosage can also cause transient bradycardia followed by tachycardia and these may be accompanied by potentially fatal cardiac arrhythmias. Premature ventricular contractions may appear within one minute after injection and may be followed by multifocal ventricular tachycardia (prefibrillation rhythm). Subsidence of the ventricular effects may be followed by atrial tachycardia and occasionally by atrioventricular block. Treatment of arrhythmias consists of administration of a beta-adrenergic blocking drug such as propranolol.
Overdosage sometimes results in extreme pallor and coldness of the skin, metabolic acidosis due to elevated blood lactic acid levels, and kidney failure. Suitable corrective measures must be taken in such situations.
Myocardial ischemia, myocardial infarction and cardiomyopathy have been noted in the literature following overdose of epinephrine.
-
11 DESCRIPTION
Adrenalin® (epinephrine injection, USP) is a clear, colorless, sterile solution containing 1 mg/mL epinephrine, packaged as 1 mL of solution in a single-use clear glass vial or 30 mL of solution in a multiple-dose amber glass vial. In the 1 mL vial, each 1 mL of Adrenalin® solution contains 1 mg epinephrine, 7.3 mg sodium chloride, 0.457 mg sodium metabisulfite, 1 mg sodium hydroxide, 2.25 mg tartaric acid, 0.20 mg disodium edetate dihydrate, hydrochloric acid to adjust pH, and water for injection. In the 30 mL vial, each 1 mL of Adrenalin® solution contains 1 mg epinephrine, 6.15 mg sodium chloride, 0.457 mg sodium metabisulfite, 0.920 mg sodium hydroxide, 2.25 mg tartaric acid, 0.20 mg disodium edetate dihydrate, hydrochloric acid to adjust pH, 5.25 mg chlorobutanol as a preservative and water for injection. The pH range is 2.2-5.0.
Epinephrine is a sympathomimetic catecholamine. The chemical name of epinephrine is: 1,2-Benzenediol, 4-[(1R)-1-hydroxy-2-(methylamino)ethyl]-, or (-)-3,4-Dihydroxy-α-[2-(methylamino)ethyl]benzyl alcohol.
The chemical structure of epinephrine is:
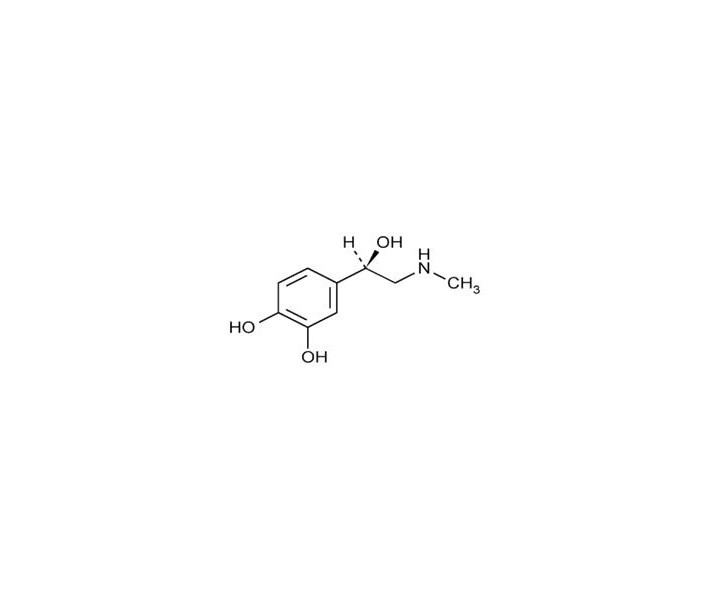
The molecular weight of epinephrine is 183.2.
Epinephrine solution deteriorates rapidly on exposure to air or light, turning pink from oxidation to adrenochrome and brown from the formation of melanin.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Epinephrine acts on both alpha and beta-adrenergic receptors.
12.2 Pharmacodynamics
Through its action on alpha-adrenergic receptors, epinephrine lessens the vasodilation and increased vascular permeability that occurs during anaphylaxis, which can lead to loss of intravascular fluid volume and hypotension.
Through its action on beta-adrenergic receptors, epinephrine causes bronchial smooth muscle relaxation and helps alleviate bronchospasm, wheezing and dyspnea that may occur during anaphylaxis.
Epinephrine also alleviates pruritus, urticaria, and angioedema and may relieve gastrointestinal and genitourinary symptoms associated with anaphylaxis because of its relaxer effects on the smooth muscle of the stomach, intestine, uterus and urinary bladder.
Epinephrine increases glycogenolysis, reduces glucose up take by tissues, and inhibits insulin release in the pancreas, resulting in hyperglycemia and increased blood lactic acid [see Warnings and Precautions 5-(5.3)].
Epinephrine causes mydriasis when administered parenterally.
12.3 Pharmacokinetics
When administered parenterally, epinephrine has a rapid onset and short duration of action.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long- term studies to evaluate the carcinogenic potential of epinephrine have not been conducted.
Epinephrine and other catecholamines have been shown to have mutagenic potential in vitro. Epinephrine was positive in the Salmonella bacterial reverse mutation assay, positive in the mouse lymphoma assay, and negative in the in vivo micronucleus assay. Epinephrine is an oxidative mutagen based on the E. coli WP2 Mutoxitest bacterial reverse mutation assay. This should not prevent the use of epinephrine under the conditions noted under Indications and Usage ( 1).
The potential for epinephrine to impair reproductive performance has not been evaluated, but epinephrine has been shown to decrease implantation in female rabbits dosed subcutaneously with 1.2 mg/kg/day (15-fold the highest human intramuscular or subcutaneous daily dose) during gestation days 3 to 9.
-
16 HOW SUPPLIED/STORAGE & HANDLING
Epinephrine Convenience Kit is supplied in the following dosage forms.
NDC 51662-1507-1 (Epinephrine Convenience Kit)
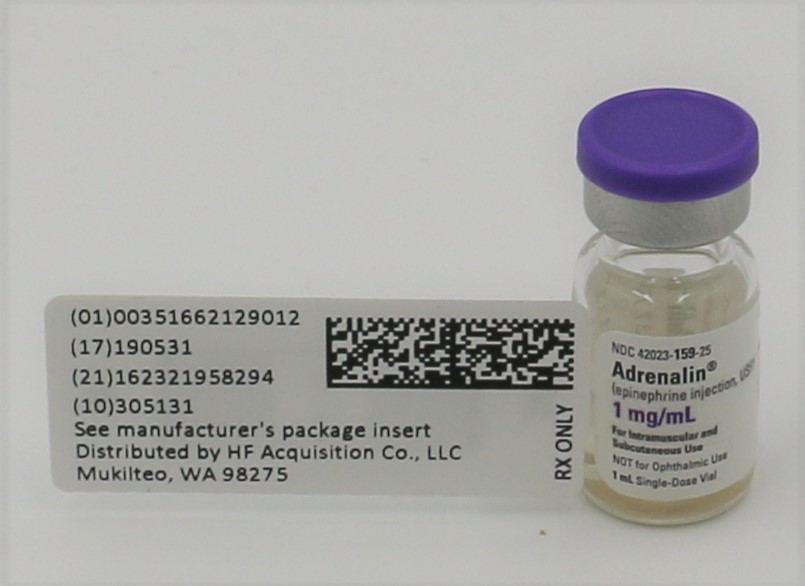
Kit Contains:Item #1007670-ADRENALIN® (EPINEPHRINE INJECTION, USP) 1mg/mL 1:1000 VIAL, NDC 51662-1290-1, 2 Total
Item # 6985-EpiRite Syringe, 2 Total
Item # 1005310-Needle 20Gx1 1/2", 2 Total
N/A-Alcohol Prep Pad, 2 Total
N/A- Package Insert, 1 Total
HF Acquisition Co LLC, DBA HealthFirst
Mukilteo, WA 98275Adrenalin® 1 mL Single-Use Vials:
Vial and contents must be discarded 30 days after initial use.
Store between 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Epinephrine is light sensitive. Protect from light and freezing.
-
17 PATIENT COUNSELING INFORMATION
Advise patients or their caregivers about common adverse reactions associated with the use of epinephrine including an increase in heart rate, the sensation of a more forceful heartbeat, palpitations, sweating, nausea and vomiting, difficulty breathing, pallor, dizziness, weakness or shakiness, headache, apprehension, nervousness, or anxiety. These symptoms and signs usually subside rapidly, especially with rest, quiet and recumbent positioning.
Warn patients with a good response to initial treatment about the possibility of recurrence of symptoms and instruct patients to obtain proper medical attention if symptoms return.
Warn patients with diabetes that they may develop increased blood glucose levels following epinephrine administration.
Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported at the injection site following epinephrine injection for anaphylaxis. Advise patients to seek medical care if they develop signs or symptoms of infection, such as persistent redness, warmth, swelling, or tenderness, at the epinephrine injection site [see Warnings and Precautions 5-(5.2)].
- SPL UNCLASSIFIED
- PRINCIPAL DISPLAY PANEL - EPINEPHRINE KIT LABELING
- PRINCIPAL DISPLAY PANEL - HYPODERMIC Needle 20Gx1 1/2" LABELING
- PRINCIPAL DISPLAY PANEL - EpiRite Syringe LABELING
- PRINCIPAL DISPLAY PANEL - Alcohol Prep Pad LABELING
- PRINCIPAL DISPLAY PANEL - VIAL LABEL
- PRINCIPAL DISPLAY PANEL - SERIALIZED VIAL LABELING
- Principal Display Panel - Serialization Example Labeling
-
INGREDIENTS AND APPEARANCE
EPINEPHRINE CONVENIENCE KIT
epinephrine kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51662-1507 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51662-1507-1 1 in 1 KIT 02/08/2020 1 1 in 1 VIAL, SINGLE-DOSE; Type 1: Convenience Kit of Co-Package Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-DOSE 1 mL Part 1 of 1 ADRENALIN (EPINEPHRINE)
adrenalin (epinephrine) injectionProduct Information Item Code (Source) NDC:51662-1290(NDC:42023-159) Route of Administration INTRAMUSCULAR, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) 1 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM METABISULFITE (UNII: 4VON5FNS3C) 0.457 mg in 1 mL WATER (UNII: 059QF0KO0R) TARTARIC ACID (UNII: W4888I119H) 2.25 mg in 1 mL EDETATE DISODIUM (UNII: 7FLD91C86K) 0.2 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 7.3 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51662-1290-1 1 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204200 02/08/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204200 02/08/2020 Labeler - HF Acquisition Co LLC, DBA HealthFirst (045657305) Registrant - HF Acquisition Co LLC, DBA HealthFirst (045657305) Establishment Name Address ID/FEI Business Operations HF Acquisition Co LLC, DBA HealthFirst 045657305 manufacture(51662-1507) , repack(51662-1290)