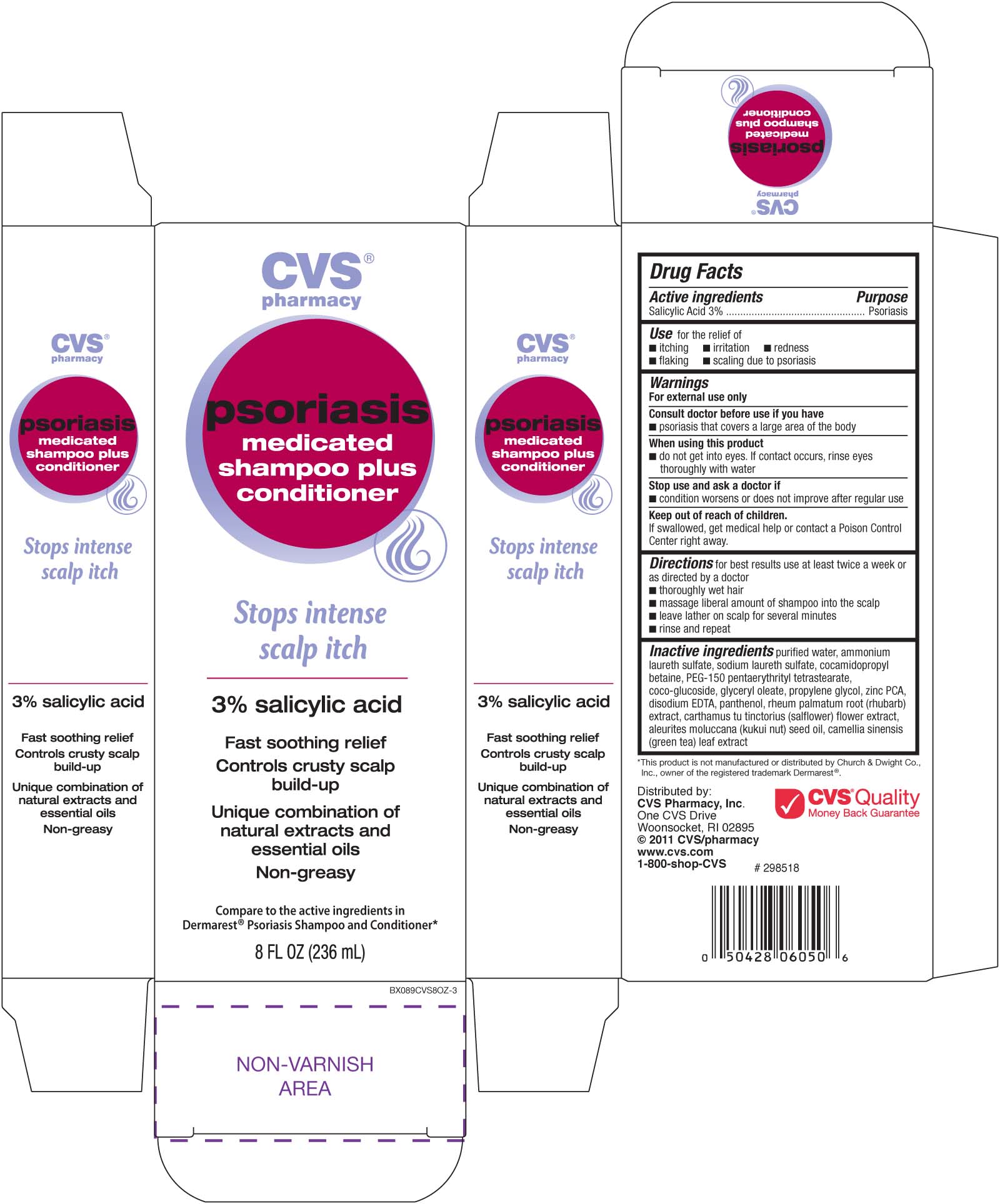
Label: CVS PSORIASIS MEDICATED- salicylic acid shampoo
-
Contains inactivated NDC Code(s)
NDC Code(s): 59779-316-63 - Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 16, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
Use for the relief of
- itching - irritation - redness - flaking - scaling due to proriasisDirections for best results use at least twice a week or as directed by a doctor
- thoroughly wet hair
- Massage liberal amount of shampoo into the scalp
- Leave lather on scalp for several minutes
- Rinse and repeat.
-
WARNINGS
Warnings For external use only.
Ask a doctor before use if you have
- psoriasis that covers a large area of the body.
When using this product
- do not get into eyes. If contact occurs, rinse eyes thoroughly with water
Stop use and ask a doctor if
- condition worsens or does not improve after regular use
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive Ingredients
purified water, ammonium Laureth sulfate, cocoamidopropyl betaine, PEG-150 pentaerythrityl tetrastearate,
coco-glucoside, glyceryl oleate, propylene glycol, zinc PCA, disodium EDTA, panthenol, rheum palmatum root
(rubarb) extract, carthamus tu tinctorius (salflower) flower extract, aleurites moluccana (kukui nut) seed oil, camellia
sinensis (green tea) leaf extract
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CVS PSORIASIS MEDICATED
salicylic acid shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-316 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) GLYCERYL MONOOLEATE (UNII: 4PC054V79P) ZINC PIDOLATE (UNII: C32PQ86DH4) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) KUKUI NUT OIL (UNII: TP11QR7B8R) PANTHENOL (UNII: WV9CM0O67Z) EDETATE DISODIUM (UNII: 7FLD91C86K) RHUBARB (UNII: G280W4MW6E) GREEN TEA LEAF (UNII: W2ZU1RY8B0) SUNFLOWER SEED (UNII: R9N3379M4Z) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-316-63 1 in 1 CARTON 1 236 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 07/16/2010 Labeler - CVS Pharmacy (062312574) Registrant - Pharma Pac, LLC (140807475) Establishment Name Address ID/FEI Business Operations Pharma Pac, LLC 140807475 manufacture