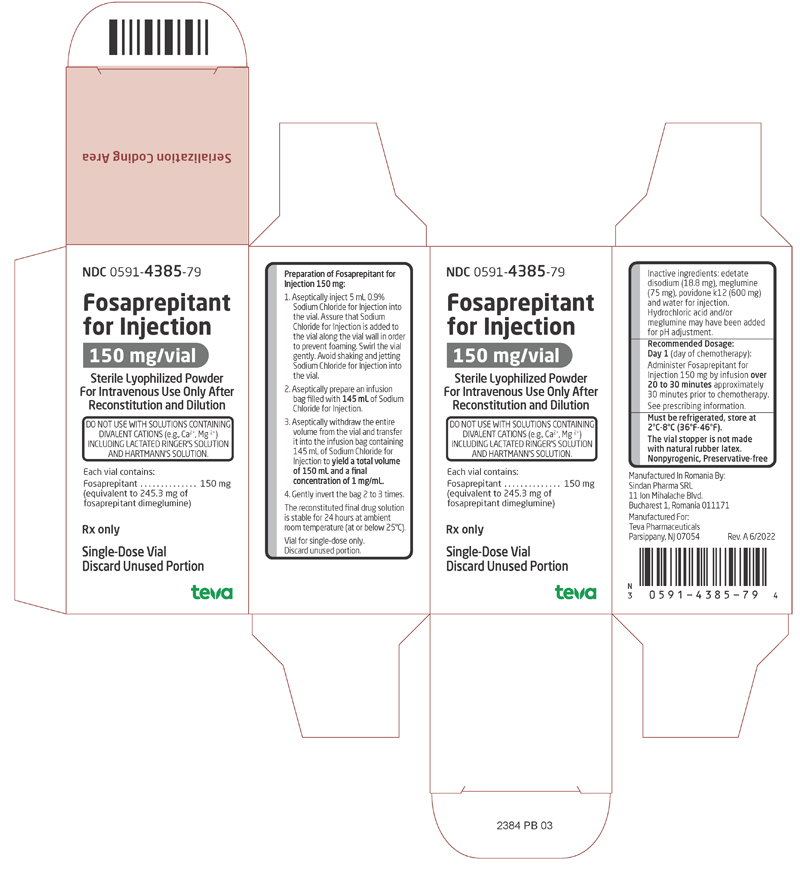
Label: FOSAPREPITANT injection, powder, lyophilized, for solution
- NDC Code(s): 0591-4385-79
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated June 30, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FOSAPREPITANT FOR INJECTION safely and effectively. See full prescribing information for FOSAPREPITANT FOR INJECTION.
FOSAPREPITANT for injection, for intravenous use
Initial U.S. Approval: 2008INDICATIONS AND USAGE
Fosaprepitant for Injection is a substance P/neurokinin-1 (NK1) receptor antagonist, indicated in adults, in combination with other antiemetic agents, for the prevention of (1):
- acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy (HEC) including high-dose cisplatin.
- delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (MEC).
Limitations of Use (1)
- Fosaprepitant for Injection has not been studied for treatment of established nausea and vomiting.
DOSAGE AND ADMINISTRATION
Dosage (2.1)
- The recommended dosage in adults is 150 mg on Day 1 as an intravenous infusion over 20 to 30 minutes completing the infusion approximately 30 minutes prior to chemotherapy.
- See Full Prescribing Information for dosages of concomitant antiemetic(s) of Fosaprepitant for Injection.
Preparation (2.2)
- Reconstitute with 5 mL of 0.9% sodium chloride.
- Add to infusion bag containing 145 mL 0.9% sodium chloride for a final concentration of 1 mg/mL.
DOSAGE FORMS AND STRENGTHS
Fosaprepitant for Injection: 150 mg fosaprepitant, lyophilized powder in single-dose vial for reconstitution. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- CYP3A4 Interactions: Fosaprepitant is a weak inhibitor of CYP3A4, and aprepitant, the active moiety, is a substrate, inhibitor, and inducer of CYP3A4; see Full Prescribing Information for recommendations regarding contraindications, risk of adverse reactions, and dosage adjustment of Fosaprepitant for Injection and concomitant drugs. (4, 5.1, 7.1, 7.2)
- Hypersensitivity Reactions (including anaphylaxis and anaphylactic shock): May occur during or soon after infusion. If symptoms occur, discontinue the drug. Do not reinitiate Fosaprepitant for Injection if symptoms occur with previous use. (4, 5.2)
- Infusion Site Reactions (including thrombophlebitis, necrosis, and vasculitis): Majority of reactions reported in patients receiving vesicant chemotherapy. Avoid infusion into small veins. Discontinue infusion and administer treatment if a severe reaction develops. (5.3)
- Warfarin (a CYP2C9 substrate): Risk of decreased INR of prothrombin time; monitor INR in 2-week period, particularly at 7 to 10 days, following initiation of Fosaprepitant for Injection. (5.4, 7.1)
- Hormonal Contraceptives: Efficacy of contraceptives may be reduced during and for 28 days following administration of Fosaprepitant for Injection. Use effective alternative or back-up methods of contraception. (5.5, 7.1, 8.3)
ADVERSE REACTIONS
- Most common adverse reactions (≥2%) are: fatigue, diarrhea, neutropenia, asthenia, anemia, peripheral neuropathy, leukopenia, dyspepsia, urinary tract infection, pain in extremity. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Teva 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Preparation Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Clinically Significant CYP3A4 Drug Interactions
5.2 Hypersensitivity Reactions
5.3 Infusion Site Reactions
5.4 Decrease in INR with Concomitant Warfarin
5.5 Risk of Reduced Efficacy of Hormonal Contraceptives
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Fosaprepitant/Aprepitant on the Pharmacokinetics of Other Drugs
7.2 Effect of Other Drugs on the Pharmacokinetics of Fosaprepitant/Aprepitant
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Prevention of Nausea and Vomiting Associated with HEC in Adults
14.2 Prevention of Nausea and Vomiting Associated with MEC in Adults
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Fosaprepitant for Injection, in combination with other antiemetic agents, is indicated in adults for the prevention of:
- acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy (HEC) including high-dose cisplatin.
- delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (MEC).
Limitations of Use
- Fosaprepitant for Injection has not been studied for the treatment of established nausea and vomiting.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of Fosaprepitant for Injection, dexamethasone, and a 5-HT3 antagonist for the prevention of nausea and vomiting associated with administration of HEC or MEC in adults is shown in Table 1 or Table 2, respectively. Administer Fosaprepitant for Injection as an intravenous infusion on Day 1 over 20 to 30 minutes, completing the infusion approximately 30 minutes prior to chemotherapy.
Table 1 Recommended Dosage for the Prevention of Nausea and Vomiting Associated with HEC Day 1
Day 2
Day 3
Day 4
Fosaprepitant for Injection
150 mg intravenously over 20 to 30 minutes
none
none
none
Dexamethasone*
12 mg orally
8 mg orally
8 mg orally twice daily
8 mg orally twice daily
5-HT3 antagonist
See selected 5-HT3 antagonist prescribing information for the recommended dosage
none
none
none
*Administer dexamethasone 30 minutes prior to chemotherapy treatment on Day 1 and in the morning on Days 2 through 4. Also administer dexamethasone in the evenings on Days 3 and 4. A 50% dosage reduction of dexamethasone on Days 1 and 2 is recommended to account for a drug interaction with Fosaprepitant for Injection [see Clinical Pharmacology (12.3)].
Table 2 Recommended Dosage for the Prevention of Nausea and Vomiting Associated with MEC Day 1
Fosaprepitant for Injection
150 mg intravenously over 20 to 30 minutes
Dexamethasone*
12 mg orally
5-HT3 antagonist
See selected 5-HT3 antagonist prescribing information for the recommended dosage
*Administer dexamethasone 30 minutes prior to chemotherapy treatment on Day 1. A 50% dosage reduction of dexamethasone is recommended to account for a drug interaction with Fosaprepitant for Injection [see Clinical Pharmacology (12.3)].
2.2 Preparation Instructions
Table 5 Preparation Instructions for Fosaprepitant for Injection (150 mg) Step 1
Aseptically inject 5 mL 0.9% Sodium Chloride Injection, USP into the vial. Assure that 0.9% Sodium Chloride Injection, USP is added to the vial along the vial wall in order to prevent foaming. Swirl the vial gently. Avoid shaking and jetting 0.9% Sodium Chloride Injection, USP into the vial.
Step 2
Aseptically prepare an infusion bag filled with 145 mL of 0.9% Sodium Chloride Injection, USP.
Step 3
Aseptically withdraw the entire volume from the vial and transfer it into the infusion bag containing 145 mL of 0.9% Sodium Chloride Injection, USP to yield a total volume of 150 mL and a final concentration of 1 mg/mL.
Step 4
Gently invert the bag 2 to 3 times.
Step 5
Before administration, inspect the bag for particulate matter and discoloration. Discard the bag if particulate and/or discoloration are observed.
Caution: Do not mix or reconstitute Fosaprepitant for Injection with solutions for which physical and chemical compatibility have not been established. Fosaprepitant for Injection is incompatible with any solutions containing divalent cations (e.g., calcium, magnesium), including Lactated Ringer’s Solution and Hartmann's Solution.
Storage
The reconstituted final drug solution is stable for 24 hours at ambient room temperature [at or below 25°C (77°F)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Fosaprepitant for Injection is contraindicated in patients:
- who are hypersensitive to any component of the product. Hypersensitivity reactions including anaphylactic reactions, flushing, erythema, and dyspnea have been reported [see Warnings and Precautions (5.2), Adverse Reactions (6.2)].
- taking pimozide. Inhibition of CYP3A4 by aprepitant, the active moiety, could result in elevated plasma concentrations of this drug, which is a CYP3A4 substrate, potentially causing serious or life-threatening reactions, such as QT prolongation, a known adverse reaction of pimozide [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Clinically Significant CYP3A4 Drug Interactions
Fosaprepitant, a prodrug of aprepitant, is a weak inhibitor of CYP3A4, and aprepitant is a substrate, inhibitor, and inducer of CYP3A4.
- Use of Fosaprepitant for Injection with other drugs that are CYP3A4 substrates, may result in increased plasma concentration of the concomitant drug.
- Use of pimozide with Fosaprepitant for Injection is contraindicated due to the risk of significantly increased plasma concentrations of pimozide, potentially resulting in prolongation of the QT interval, a known adverse reaction of pimozide [see Contraindications (4)].
- Use of Fosaprepitant for Injection with strong or moderate CYP3A4 inhibitors (e.g., ketoconazole, diltiazem) may increase plasma concentrations of aprepitant and result in an increased risk of adverse reactions related to Fosaprepitant for Injection.
- Use of Fosaprepitant for Injection with strong CYP3A4 inducers (e.g., rifampin) may result in a reduction in aprepitant plasma concentrations and decreased efficacy of Fosaprepitant for Injection.
See Table 7 and Table 8 for a listing of potentially significant drug interactions [see Drug Interactions (7.1, 7.2)].
5.2 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis and anaphylactic shock, during or soon after infusion of fosaprepitant have occurred. Symptoms including flushing, erythema, dyspnea, hypotension and syncope have been reported [see Adverse Reactions (6.2)].
Monitor patients during and after infusion. If hypersensitivity reactions occur, discontinue the infusion and administer appropriate medical therapy. Do not reinitiate Fosaprepitant for Injection in patients who experience these symptoms with previous use [see Contraindications (4)].
5.3 Infusion Site Reactions
Infusion site reactions (ISRs) have been reported with the use of Fosaprepitant for Injection [see Adverse Reactions (6.1)]. The majority of severe ISRs, including thrombophlebitis and vasculitis, were reported with concomitant vesicant (anthracycline-based) chemotherapy administration, particularly when associated with extravasation. Necrosis was also reported in some patients with concomitant vesicant chemotherapy. Most ISRs occurred with the first, second or third exposure to single doses of Fosaprepitant for Injection and in some cases, reactions persisted for two weeks or longer. Treatment of severe ISRs consisted of medical, and in some cases surgical, intervention.
Avoid infusion of Fosaprepitant for Injection into small veins or through a butterfly catheter. If a severe ISR develops during infusion, discontinue the infusion and administer appropriate medical treatment.
5.4 Decrease in INR with Concomitant Warfarin
Coadministration of Fosaprepitant for Injection with warfarin, a CYP2C9 substrate, may result in a clinically significant decrease in the International Normalized Ratio (INR) of prothrombin time [see Clinical Pharmacology (12.3)]. Monitor the INR in patients on chronic warfarin therapy in the 2-week period, particularly at 7 to 10 days, following initiation of Fosaprepitant for Injection with each chemotherapy cycle [see Drug Interactions (7.1)].
5.5 Risk of Reduced Efficacy of Hormonal Contraceptives
Upon coadministration with Fosaprepitant for Injection, the efficacy of hormonal contraceptives may be reduced during administration of and for 28 days following the last dose of Fosaprepitant for Injection [see Clinical Pharmacology (12.3)]. Advise patients to use effective alternative or back-up methods of contraception during treatment with Fosaprepitant for Injection and for 1 month following administration of Fosaprepitant for Injection [see Drug Interactions (7.1), Use in Specific Populations (8.3)].
- Use of Fosaprepitant for Injection with other drugs that are CYP3A4 substrates, may result in increased plasma concentration of the concomitant drug.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
- Infusion Site Reactions [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of Fosaprepitant for Injection has been established from adequate and well-controlled studies of another intravenous fosaprepitant product in chemotherapy induced nausea and vomiting [see Clinical Studies (14)]. Adverse reactions reported in these studies are described below.
Adverse Reactions for the Prevention of Nausea and Vomiting Associated with MEC
In an active-controlled clinical trial in patients receiving MEC, safety was evaluated in 504 adult patients receiving a single intravenous dose of fosaprepitant in combination with ondansetron and dexamethasone (fosaprepitant regimen) compared to 497 patients receiving ondansetron and dexamethasone alone (standard therapy). The most common adverse reactions are listed in Table 6.
Table 6 Most Common Adverse Reactions in Patients Receiving MEC* Intravenous fosaprepitant, ondansetron, and dexamethasone†
(N=504)
Ondansetron and dexamethasone‡
(N=497)
fatigue
15%
13%
diarrhea
13%
11%
neutropenia
8%
7%
asthenia
4%
3%
anemia
3%
2%
peripheral neuropathy
3%
2%
leukopenia
2%
1%
dyspepsia
2%
1%
urinary tract infection
2%
1%
pain in extremity
2%
1%
* Reported in ≥2% of patients treated with the fosaprepitant regimen and at a greater incidence than standard therapy.
†Fosaprepitant regimen
‡Standard therapy
Infusion-site reactions were reported in 2.2% of patients treated with the fosaprepitant regimen compared to 0.6% of patients treated with standard therapy. The infusion-site reactions included: infusion-site pain (1.2%, 0.4%), injection-site irritation (0.2%, 0.0%), vessel puncture-site pain (0.2%, 0.0%), and infusion-site thrombophlebitis (0.6%, 0.0%), reported in the fosaprepitant regimen compared to standard therapy, respectively.
Adverse Reactions for the Prevention of Nausea and Vomiting Associated with HEC
In an active-controlled clinical study in patients receiving HEC, safety was evaluated for 1143 adult patients receiving a single intravenous dose of fosaprepitant compared to 1169 patients receiving the 3-day regimen of oral aprepitant [see Clinical Studies (14.1)]. The safety profile was generally similar to that seen in the MEC study with fosaprepitant and prior HEC studies with aprepitant. However, infusion-site reactions occurred at a higher incidence in patients in the fosaprepitant group (3.0%) compared to those in the aprepitant group (0.5%). The following additional infusion-site reactions occurred in the HEC study and were not reported in the MEC study described above: infusion-site erythema (0.5%, 0.1%), infusion-site pruritus (0.3%, 0.0%), and infusion-site induration (0.2%, 0.1%), reported in the fosaprepitant group compared to the aprepitant group, respectively.
Because fosaprepitant is converted to aprepitant, those adverse reactions associated with aprepitant might also be expected to occur with Fosaprepitant for Injection. See the full prescribing information for aprepitant capsules for complete safety information regarding studies performed with oral aprepitant.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of another intravenous formulation of fosaprepitant. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and subcutaneous tissue disorders: pruritus, rash, urticaria, Stevens-Johnson syndrome/toxic epidermal necrolysis [see Warnings and Precautions (5.2)].
Immune system disorders: hypersensitivity reactions including anaphylaxis and anaphylactic shock [see Contraindications (4), Warnings and Precautions (5.2)].
Nervous system disorders: ifosfamide-induced neurotoxicity reported after fosaprepitant and ifosfamide coadministration.
-
7 DRUG INTERACTIONS
7.1 Effect of Fosaprepitant/Aprepitant on the Pharmacokinetics of Other Drugs
When administered intravenously, fosaprepitant, a prodrug of aprepitant, is converted to aprepitant within 30 minutes. Therefore, drug interactions following administration of Fosaprepitant for Injection are likely to occur with drugs that interact with oral aprepitant.
Fosaprepitant, given as a single 150-mg dose, is a weak inhibitor of CYP3A4, and the weak inhibition of CYP3A4 continues for 2 days after single dose administration. Single dose fosaprepitant does not induce CYP3A4. Aprepitant is a substrate, an inhibitor, and an inducer of CYP3A4. Aprepitant is also an inducer of CYP2C9 [see Clinical Pharmacology (12.3)].
Some substrates of CYP3A4 are contraindicated with Fosaprepitant for Injection [see Contraindications (4)]. Dosage adjustment of some CYP3A4 and CYP2C9 substrates may be warranted, as shown in Table 7.
Table 7 Effects of Fosaprepitant/Aprepitant on the Pharmacokinetics of Other Drugs CYP3A4 Substrates
Pimozide
Clinical Impact
Increased pimozide exposure
Intervention
Fosaprepitant for Injection is contraindicated [see Contraindications (4)].
Benzodiazepines
Clinical Impact
Increased exposure to midazolam or other benzodiazepines metabolized via CYP3A4 (alprazolam, triazolam) may increase the risk of adverse reactions [see Clinical Pharmacology (12.3)].
Intervention
Monitor for benzodiazepine-related adverse reactions.
Dexamethasone
Clinical Impact
Increased dexamethasone exposure [see Clinical Pharmacology (12.3)].
Intervention
Reduce the dose of oral dexamethasone by approximately 50% [see Dosage and Administration (2.1)].
Methylprednisolone
Clinical Impact
Increased methylprednisolone exposure [see Clinical Pharmacology (12.3)].
Intervention
Reduce the dose of oral methylprednisolone by approximately 50% on Days 1 and 2 for patients receiving HEC and on Day 1 for patients receiving MEC.
Reduce the dose of intravenous methylprednisolone by 25% on Days 1 and 2 for patients receiving HEC and on Day 1 for patients receiving MEC.
Chemotherapeutic agents that are metabolized by CYP3A4
Clinical Impact
Increased exposure of the chemotherapeutic agent may increase the risk of adverse reactions [see Clinical Pharmacology (12.3)].
Intervention
Vinblastine, vincristine, or ifosfamide or other chemotherapeutic agents
- Monitor for chemotherapeutic-related adverse reactions.
Etoposide, vinorelbine, paclitaxel, and docetaxel
- No dosage adjustment needed.
Hormonal Contraceptives
Clinical Impact
Decreased hormonal exposure during administration of and for 28 days after administration of the last dose of Fosaprepitant for Injection [see Warnings and Precautions (5.5), Use in Specific Populations (8.3), Clinical Pharmacology (12.3)].
Intervention
Effective alternative or back-up methods of contraception (such as condoms or spermicides) should be used during treatment with Fosaprepitant for Injection and for 1 month following administration of Fosaprepitant for Injection.
Examples
birth control pills, skin patches, implants, and certain IUDs
CYP2C9 Substrates
Warfarin
Clinical Impact
Decreased warfarin exposure and decreased prothrombin time (INR) [see Warnings and Precautions (5.4), Clinical Pharmacology (12.3)].
Intervention
In patients on chronic warfarin therapy, monitor the prothrombin time (INR) in the 2-week period, particularly at 7 to 10 days, following administration of Fosaprepitant for Injection with each chemotherapy cycle.
Other
5-HT3 Antagonists
Clinical Impact
No change in the exposure of the 5-HT3 antagonist [see Clinical Pharmacology (12.3)].
Intervention
No dosage adjustment needed
Examples
ondansetron, granisetron, dolasetron
7.2 Effect of Other Drugs on the Pharmacokinetics of Fosaprepitant/Aprepitant
Aprepitant is a CYP3A4 substrate [see Clinical Pharmacology (12.3)]. Coadministration of Fosaprepitant for Injection with drugs that are inhibitors or inducers of CYP3A4 may result in increased or decreased plasma concentrations of aprepitant, respectively, as shown in Table 8.
Table 8 Effects of Other Drugs on Pharmacokinetics of Fosaprepitant/Aprepitant Moderate to Strong CYP3A4 Inhibitors
Clinical Impact
Significantly increased exposure of aprepitant may increase the risk of adverse reactions associated with Fosaprepitant for Injection [see Adverse Reactions (6.1), Clinical Pharmacology (12.3)].
Intervention
Avoid concomitant use of Fosaprepitant for Injection
Examples
Moderate inhibitor:
diltiazemStrong inhibitors:
ketoconazole, itraconazole, nefazodone, troleandomycin, clarithromycin, ritonavir, nelfinavir
Strong CYP3A4 Inducers
Clinical Impact
Substantially decreased exposure of aprepitant in patients chronically taking a strong CYP3A4 inducer may decrease the efficacy of Fosaprepitant for Injection [see Clinical Pharmacology (12.3)].
Intervention
Avoid concomitant use of Fosaprepitant for Injection
Examples
rifampin, carbamazepine, phenytoin
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on use of fosaprepitant in pregnant women to inform a drug-associated risk of adverse developmental outcomes. In animal reproduction studies, no adverse developmental effects were observed in rats or rabbits exposed during the period of organogenesis to systemic exposures (AUC) approximately equivalent to the exposure at the recommended human dose (RHD) of 150 mg [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In embryofetal development studies in rats and rabbits, aprepitant was administered during the period of organogenesis at oral doses up to 1,000 mg/kg twice daily (rats) and up to the maximum tolerated dose of 25 mg/kg/day (rabbits). No embryofetal lethality or malformations were observed at any dose level in either species. The exposures (AUC) in pregnant rats at 1,000 mg/kg twice daily and in pregnant rabbits at 25 mg/kg/day were approximately equivalent to the exposure at the RHD of 150 mg. Aprepitant crosses the placenta in rats and rabbits.
8.2 Lactation
Risk Summary
There are no data on the presence of aprepitant in human milk, the effects on the breastfed infant, or the effects on milk production. Aprepitant is present in rat milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Fosaprepitant for Injection and any potential adverse effects on the breastfed infant from Fosaprepitant for Injection or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Contraception
Upon administration of Fosaprepitant for Injection, the efficacy of hormonal contraceptives may be reduced. Advise females of reproductive potential using hormonal contraceptives to use an effective alternative or back-up non-hormonal contraceptive (such as condoms or spermicides) during treatment with Fosaprepitant for Injection and for 1 month following the last dose [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
8.4 Pediatric Use
This Fosaprepitant for Injection product is not approved for use in pediatric patients.
This Fosaprepitant for Injection product contains 18.8 mg of edetate disodium (EDTA) per vial [see Description (11)]. Edetate disodium is a chelator of metal ions, including calcium. Other fosaprepitant for injection products may contain less edetate disodium.
8.5 Geriatric Use
Of the 1649 adult cancer patients treated with intravenous fosaprepitant in HEC and MEC clinical studies, 27% were aged 65 and over, while 5% were aged 75 and over. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, use caution when dosing elderly patients as they have a greater frequency of decreased hepatic, renal or cardiac function and concomitant disease or other drug therapy [see Clinical Pharmacology (12.3)].
8.6 Patients with Hepatic Impairment
The pharmacokinetics of aprepitant in patients with mild and moderate hepatic impairment were similar to those of healthy subjects with normal hepatic function. No dosage adjustment is necessary for patients with mild to moderate hepatic impairment (Child-Pugh score 5 to 9). There are no clinical or pharmacokinetic data in patients with severe hepatic impairment (Child-Pugh score greater than 9). Therefore, additional monitoring for adverse reactions in these patients may be warranted when Fosaprepitant for Injection is administered [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
There is no specific information on the treatment of overdosage with fosaprepitant or aprepitant.
In the event of overdose, Fosaprepitant for Injection should be discontinued and general supportive treatment and monitoring should be provided. Because of the antiemetic activity of Fosaprepitant for Injection, drug-induced emesis may not be effective in cases of Fosaprepitant for Injection overdosage.
Aprepitant is not removed by hemodialysis.
-
11 DESCRIPTION
Fosaprepitant for Injection is a sterile, lyophilized formulation containing fosaprepitant dimeglumine, a prodrug of aprepitant, a substance P/neurokinin-1 (NK1) receptor antagonist, an antiemetic agent, chemically described as 1-Deoxy-1-(methylamino)-D-glucitol[3-[[(2R,3S)-2-[(1R)-1-[3,5- bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-2,5-dihydro-5-oxo-1H-1,2,4- triazol-1-yl]phosphonate (2:1) (salt).
Its empirical formula is C23H22F7N4O6P • 2(C7H17NO5) and its structural formula is:
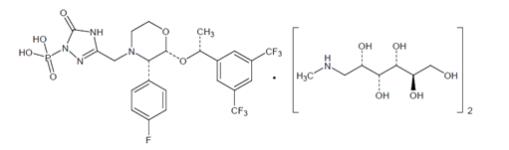
Fosaprepitant dimeglumine is a white to off-white amorphous powder with a molecular weight of 1004.83. It is freely soluble in water.
Each vial of Fosaprepitant for Injection for administration as an intravenous infusion contains 150 mg of fosaprepitant (equivalent to 245.3 mg of fosaprepitant dimeglumine) and the following inactive ingredients: edetate disodium (18.8 mg), meglumine (75 mg), povidone k12 (600 mg), and water for injection. Hydrochloric acid and/or meglumine may have been added for pH adjustment.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fosaprepitant is a prodrug of aprepitant and accordingly, its antiemetic effects are attributable to aprepitant.
Aprepitant is a selective high-affinity antagonist of human substance P/neurokinin 1 (NK1) receptors. Aprepitant has little or no affinity for serotonin (5-HT3), dopamine, and corticosteroid receptors, the targets of existing therapies for chemotherapy-induced nausea and vomiting (CINV). Aprepitant has been shown in animal models to inhibit emesis induced by cytotoxic chemotherapeutic agents, such as cisplatin, via central actions. Animal and human Positron Emission Tomography (PET) studies with aprepitant have shown that it crosses the blood brain barrier and occupies brain NK1 receptors. Animal and human studies have shown that aprepitant augments the antiemetic activity of the 5-HT3-receptor antagonist ondansetron and the corticosteroid dexamethasone and inhibits both the acute and delayed phases of cisplatin-induced emesis.
12.2 Pharmacodynamics
Cardiac Electrophysiology
In a randomized, double-blind, positive-controlled, thorough QTc study, a single 200-mg dose of fosaprepitant (approximately 1.3 times the recommended dose) had no effect on the QTc interval.
12.3 Pharmacokinetics
Aprepitant after Fosaprepitant Administration
Following administration of a single intravenous 150-mg dose of fosaprepitant, a prodrug of aprepitant administered as a 20-minute infusion to healthy subjects, the mean AUC0-∞ of aprepitant was 37.4 (± 14.8) mcg•hr/mL and the mean maximal aprepitant concentration (Cmax) was 4.2 (± 1.2) mcg/mL. Plasma concentrations of fosaprepitant are below the limits of quantification (10 ng/mL) within 30 minutes of the completion of infusion.
Distribution
Aprepitant is greater than 95% bound to plasma proteins. The mean apparent volume of distribution at steady state (Vdss) was approximately 70 L in humans.
Aprepitant crosses the blood brain barrier in humans [see Clinical Pharmacology (12.1)].
Elimination
Metabolism
Fosaprepitant is converted to aprepitant in in vitro incubations with human liver preparations and in S9 preparations from multiple other human tissues including kidney, lung and ileum. Thus, it appears that the conversion of fosaprepitant to aprepitant can occur in multiple extrahepatic tissues in addition to the liver.
Aprepitant undergoes extensive metabolism. In vitro studies using human liver microsomes indicate that aprepitant is metabolized primarily by CYP3A4 with minor metabolism by CYP1A2 and CYP2C19. Metabolism is largely via oxidation at the morpholine ring and its side chains. No metabolism by CYP2D6, CYP2C9, or CYP2E1 was detected.
In healthy young adults, aprepitant accounts for approximately 24% of the radioactivity in plasma over 72 hours following a single oral 300-mg dose of [14C]-aprepitant, indicating a substantial presence of metabolites in the plasma. Seven metabolites of aprepitant, which are only weakly active, have been identified in human plasma.
Excretion
Following administration of a single intravenous 100-mg dose of [14C]-fosaprepitant to healthy subjects, 57% of the radioactivity was recovered in urine and 45% in feces.
Aprepitant is eliminated primarily by metabolism; aprepitant is not renally excreted. The apparent terminal half-life ranged from approximately 9 to 13 hours.
Specific Populations
Geriatric Patients
Following oral administration of a single 125-mg dose of aprepitant on Day 1 and 80 mg once daily on Days 2 through 5, the AUC0-24hr of aprepitant was 21% higher on Day 1 and 36% higher on Day 5 in elderly (65 years and older) relative to younger adults. The Cmax was 10% higher on Day 1 and 24% higher on Day 5 in elderly relative to younger adults. These differences are not considered clinically meaningful [see Use in Specific Populations (8.5)].
Male and Female Patients
Following oral administration of a single dose of aprepitant, ranging from 40 mg to 375 mg, the AUC0-24hr and Cmax are 9% and 17% higher in females as compared with males. The half-life of aprepitant is approximately 25% lower in females as compared with males and Tmax occurs at approximately the same time. These differences are not considered clinically meaningful.
Racial or Ethnic Groups
Following oral administration of a single dose of aprepitant, ranging from 40 mg to 375 mg, the AUC0-24hr and Cmax are approximately 27% and 19% higher in Hispanics as compared with Caucasians. The AUC0-24hr and Cmax were 74% and 47% higher in Asians as compared to Caucasians. There was no difference in AUC0-24hr or Cmax between Caucasians and Blacks. These differences are not considered clinically meaningful.
Patients with Renal Impairment
A single 240-mg oral dose of aprepitant was administered to patients with severe renal impairment (creatinine clearance less than 30 mL/min/1.73 m2 as measured by 24-hour urinary creatinine clearance) and to patients with end stage renal disease (ESRD) requiring hemodialysis.
In patients with severe renal impairment, the AUC0-∞ of total aprepitant (unbound and protein bound) decreased by 21% and Cmax decreased by 32%, relative to healthy subjects (creatinine clearance greater than 80 mL/min estimated by Cockcroft-Gault method). In patients with ESRD undergoing hemodialysis, the AUC0-∞ of total aprepitant decreased by 42% and Cmax decreased by 32%. Due to modest decreases in protein binding of aprepitant in patients with renal disease, the AUC of pharmacologically active unbound drug was not significantly affected in patients with renal impairment compared with healthy subjects. Hemodialysis conducted 4 or 48 hours after dosing had no significant effect on the pharmacokinetics of aprepitant; less than 0.2% of the dose was recovered in the dialysate.
Patients with Hepatic Impairment
Fosaprepitant is metabolized in various extrahepatic tissues; therefore hepatic impairment is not expected to alter the conversion of fosaprepitant to aprepitant.
Following administration of a single 125-mg oral dose of aprepitant on Day 1 and 80 mg once daily on Days 2 and 3 to patients with mild hepatic impairment (Child-Pugh score 5 to 6), the AUC0-24hr of aprepitant was 11% lower on Day 1 and 36% lower on Day 3, as compared with healthy subjects given the same regimen. In patients with moderate hepatic impairment (Child-Pugh score 7 to 9), the AUC0-24hr of aprepitant was 10% higher on Day 1 and 18% higher on Day 3, as compared with healthy subjects given the same regimen. These differences in AUC0-24hr are not considered clinically meaningful. There are no clinical or pharmacokinetic data in patients with severe hepatic impairment (Child-Pugh score greater than 9) [see Use in Specific Populations (8.6)].
Body Mass Index (BMI)
For every 5 kg/m2 increase in BMI, AUC0-24hr and Cmax of aprepitant decrease by 9% and 10%. BMI of subjects in the analysis ranged from 18 kg/m2 to 36 kg/m2. This change is not considered clinically meaningful.
Drug Interactions Studies
Fosaprepitant, given as a single 150-mg dose, is a weak inhibitor of CYP3A4, with no evidence of inhibition or induction of CYP3A4 observed on Day 4. The weak inhibition of CYP3A4 continues for 2 days after single dose administration of fosaprepitant. Aprepitant is a substrate, an inhibitor, and an inducer of CYP3A4. Aprepitant is also an inducer of CYP2C9.
Fosaprepitant or aprepitant is unlikely to interact with drugs that are substrates for the P-glycoprotein transporter.
Effects of Fosaprepitant/Aprepitant on the Pharmacokinetics of Other Drugs
CYP3A4 Substrates
Midazolam: Fosaprepitant 150 mg administered as a single intravenous dose on Day 1 increased the AUC0-∞ of midazolam by approximately 1.8-fold on Day 1 and had no effect on Day 4 when midazolam was coadministered as a single oral dose of 2 mg on Days 1 and 4 [see Drug Interactions (7.1)].
Corticosteroids:
Dexamethasone: Fosaprepitant administered as a single 150 mg intravenous dose on Day 1 increased the AUC0-24hr of dexamethasone, administered as a single 8-mg oral dose on Days 1, 2, and 3, by approximately 2-fold on Days 1 and 2 [see Dosage and Administration (2.1), Drug Interactions (7.1)].
Methylprednisolone: When oral aprepitant as a 3-day regimen (125-mg/80-mg/80-mg) was administered with intravenous methylprednisolone 125 mg on Day 1 and oral methylprednisolone 40 mg on Days 2 and 3, the AUC of methylprednisolone was increased by 1.34-fold on Day 1 and by 2.5-fold on Day 3 [see Drug Interactions (7.1)].
Chemotherapeutic agents:
Docetaxel: In a pharmacokinetic study, oral aprepitant administered as a 3-day regimen (125-mg/80-mg/80-mg) did not influence the pharmacokinetics of docetaxel.
Vinorelbine: In a pharmacokinetic study, oral aprepitant administered as a 3-day regimen (125-mg/80-mg/80-mg) did not influence the pharmacokinetics of vinorelbine to a clinically significant degree.
Oral contraceptives: When oral aprepitant was administered as a 3-day regimen (125-mg/80-mg/80-mg) with ondansetron and dexamethasone, and coadministered with an oral contraceptive containing ethinyl estradiol and norethindrone, the trough concentrations of both ethinyl estradiol and norethindrone were reduced by as much as 64% for 3 weeks post-treatment [see Drug Interactions (7.1)].
CYP2C9 substrates (Warfarin, Tolbutamide):
Warfarin: A single 125-mg dose of oral aprepitant was administered on Day 1 and 80 mg/day on Days 2 and 3 to subjects who were stabilized on chronic warfarin therapy. Although there was no effect of oral aprepitant on the plasma AUC of R(+) or S(-) warfarin determined on Day 3, there was a 34% decrease in S(-) warfarin trough concentration accompanied by a 14% decrease in the prothrombin time (reported as International Normalized Ratio or INR) 5 days after completion of dosing with oral aprepitant [see Drug Interactions (7.1)].
Tolbutamide: Oral aprepitant, when given as 125 mg on Day 1 and 80 mg/day on Days 2 and 3, decreased the AUC of tolbutamide by 23% on Day 4, 28% on Day 8, and 15% on Day 15, when a single dose of tolbutamide 500 mg was administered prior to the administration of the 3-day regimen of oral aprepitant and on Days 4, 8, and 15. This effect was not considered clinically important.
Other Drugs
P-glycoprotein substrates: Aprepitant is unlikely to interact with drugs that are substrates for the P-glycoprotein transporter, as demonstrated by the lack of interaction of oral aprepitant with digoxin in a clinical drug interaction study.
5-HT3 antagonists: In clinical drug interaction studies, aprepitant did not have clinically important effects on the pharmacokinetics of ondansetron, granisetron, or hydrodolasetron (the active metabolite of dolasetron).
Effect of Other Drugs on the Pharmacokinetics of Fosaprepitant/Aprepitant
Rifampin: When a single 375-mg dose of oral aprepitant was administered on Day 9 of a 14-day regimen of
600 mg/day of rifampin, a strong CYP3A4 inducer, the AUC of aprepitant decreased approximately 11-fold and the mean terminal half-life decreased approximately 3-fold [see Drug Interactions (7.2)].Ketoconazole: When a single 125-mg dose of oral aprepitant was administered on Day 5 of a 10-day regimen of 400 mg/day of ketoconazole, a strong CYP3A4 inhibitor, the AUC of aprepitant increased approximately 5-fold and the mean terminal half-life of aprepitant increased approximately 3-fold [see Drug Interactions (7.2)].
Diltiazem: In a study in 10 patients with mild to moderate hypertension, administration of 100 mg of fosaprepitant as an intravenous infusion with 120 mg of diltiazem, a moderate CYP3A4 inhibitor administered three times daily, resulted in a 1.5-fold increase in the aprepitant AUC and a 1.4-fold increase in the diltiazem AUC.
When fosaprepitant was administered with diltiazem, the mean maximum decrease in diastolic blood pressure was significantly greater than that observed with diltiazem alone [24.3 ± 10.2 mm Hg with fosaprepitant versus 15.6 ± 4.1 mm Hg without fosaprepitant]. The mean maximum decrease in systolic blood pressure was also greater after co-administration of diltiazem with fosaprepitant than administration of diltiazem alone [29.5 ± 7.9 mm Hg with fosaprepitant versus 23.8 ± 4.8 mm Hg without fosaprepitant]. Coadministration of fosaprepitant and diltiazem; however, did not result in any additional clinically significant changes in heart rate or PR interval, beyond those changes observed with diltiazem alone [see Drug Interactions (7.2)].
Paroxetine: Coadministration of once daily doses of oral aprepitant 170 mg, with paroxetine 20 mg once daily, resulted in a decrease in AUC by approximately 25% and Cmax by approximately 20% of both aprepitant and paroxetine. This effect was not considered clinically important.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies were conducted in Sprague-Dawley rats and in CD-1 mice for 2 years. In the rat carcinogenicity studies, animals were treated with oral doses ranging from 0.05 to 1,000 mg/kg twice daily. The highest dose produced systemic exposures to aprepitant approximately equivalent to (female rats) or less than (male rats) the adult human exposure at the RHD of 150 mg. Treatment with aprepitant at doses of 5 to 1,000 mg/kg twice daily caused an increase in the incidences of thyroid follicular cell adenomas and carcinomas in male rats. In female rats, it produced hepatocellular adenomas at 5 to 1,000 mg/kg twice daily and hepatocellular carcinomas and thyroid follicular cell adenomas at 125 to 1,000 mg/kg twice daily. In the mouse carcinogenicity studies, the animals were treated with oral doses ranging from 2.5 to 2,000 mg/kg/day. The highest dose produced a systemic exposure approximately 2 times the adult human exposure at the RHD of 150 mg. Treatment with aprepitant produced skin fibrosarcomas at 125 and 500 mg/kg/day doses in male mice. Carcinogenicity studies were not conducted with fosaprepitant.
Mutagenesis
Aprepitant and fosaprepitant were not genotoxic in the Ames test, the human lymphoblastoid cell (TK6) mutagenesis test, the rat hepatocyte DNA strand break test, the Chinese hamster ovary (CHO) cell chromosome aberration test and the mouse micronucleus test.
Impairment of Fertility
Fosaprepitant, when administered intravenously, is rapidly converted to aprepitant. In the fertility studies conducted with fosaprepitant and aprepitant, the highest systemic exposures to aprepitant were obtained following oral administration of aprepitant. Oral aprepitant did not affect the fertility or general reproductive performance of male or female rats at doses up to the maximum feasible dose of 1,000 mg/kg twice daily (providing exposure in male rats lower than the exposure at the recommended adult human dose of 150 mg and exposure in female rats approximately equivalent to the adult human exposure).
-
14 CLINICAL STUDIES
The safety and efficacy of Fosaprepitant for Injection have been established based on adequate and well-controlled adult studies of another intravenous fosaprepitant product in chemotherapy induced nausea and vomiting. Below is a display of the results of these adequate and well-controlled studies of fosaprepitant in these conditions.
14.1 Prevention of Nausea and Vomiting Associated with HEC in Adults
In a randomized, parallel, double-blind, active-controlled study, 150 mg fosaprepitant as a single intravenous infusion (N=1147) was compared to a 3-day oral aprepitant capsules regimen (N=1175) in patients receiving a HEC regimen that included cisplatin (≥70 mg/m2). All patients in both groups received dexamethasone and ondansetron (see Table 11). Patient demographics were similar between the two treatment groups. Of the total 2322 patients, 63% were men, 56% White, 26% Asian, 3% American Indian/Alaska Native, 2% Black, 13% Multi-Racial, and 33% Hispanic/Latino ethnicity. Patient ages ranged from 19 to 86 years of age, with a mean age of 56 years. Other concomitant chemotherapy agents commonly administered were fluorouracil (17%), gemcitabine (16%), paclitaxel (15%), and etoposide (12%).
Table 11 Treatment Regimens in Adult HEC Trial* Day 1
Day 2
Day 3
Day 4
Intravenous Fosaprepitant Regimen
Fosaprepitant
150 mg intravenously over 20 to 30 minutes approximately 30 minutes prior to chemotherapy
none
none
none
Oral dexamethasone†
12 mg
8 mg
8 mg twice daily
8 mg twice daily
Ondansetron
Ondansetron‡
none
none
none
Oral Aprepitant Regimen
Aprepitant capsules
125 mg
80 mg
80 mg
none
Oral dexamethasone§
12 mg
8 mg
8 mg
8 mg
Ondansetron
Ondansetron‡
none
none
none
*Fosaprepitant placebo, aprepitant capsules placebo and dexamethasone placebo (in the evenings on Days 3 and 4) were used to maintain blinding.
†Dexamethasone was administered 30 minutes prior to chemotherapy treatment on Day 1 and in the morning on Days 2 through 4. Dexamethasone was also administered in the evenings on Days 3 and 4. The 12 mg dose of dexamethasone on Day 1 and the 8 mg once daily dose on Day 2 reflects a dosage adjustment to account for a drug interaction with the fosaprepitant regimen [see Clinical Pharmacology (12.3)].
‡Ondansetron 32 mg intravenous was used in the clinical trials of fosaprepitant. Although this dose was used in clinical trials, this is no longer the currently recommended dose. Refer to the ondansetron prescribing information for the current recommended dose.
§Dexamethasone was administered 30 minutes prior to chemotherapy treatment on Day 1 and in the morning on Days 2 through 4. The 12 mg dose of dexamethasone on Day 1 and the 8 mg once daily dose on Days 2 through 4 reflects a dosage adjustment to account for a drug interaction with the oral aprepitant capsules regimen [see Clinical Pharmacology (12.3)].
The efficacy of a single-dose of intravenous fosaprepitant was evaluated based on the primary and secondary endpoints listed in Table 12 and was shown to be non-inferior to that of the 3-day oral aprepitant regimen with regard to complete response in each of the evaluated phases. The pre-specified non-inferiority margin for complete response in the overall phase was 7%. The pre-specified non-inferiority margin for complete response in the delayed phase was 7.3%. The pre-specified non-inferiority margin for no vomiting in the overall phase was 8.2%.
Table 12 Percent of Adult Patients Receiving HEC Responding by Treatment Group and Phase - Cycle 1 ENDPOINTS
Intravenous Fosaprepitant Regimen
Oral Aprepitant Regimen
Difference†
(N=1106)*
(N=1134)*
(95% CI)
%
%
PRIMARY ENDPOINT
Complete Response‡
Overall§
71.9
72.3
-0.4 (-4.1, 3.3)
SECONDARY ENDPOINTS
Complete Response‡
Delayed phase¶
74.3
74.2
0.1 (-3.5, 3.7)
No Vomiting
Overall§
72.9
74.6
-1.7 (-5.3, 2.0)
*N: Number of patients included in the primary analysis of complete response.
†Difference and Confidence interval (CI) were calculated using the method proposed by Miettinen and Nurminen and adjusted for Gender.
‡Complete Response = no vomiting and no use of rescue therapy.
§Overall = 0 to 120 hours post-initiation of cisplatin chemotherapy.
¶Delayed phase = 25 to 120 hours post-initiation of cisplatin chemotherapy.
14.2 Prevention of Nausea and Vomiting Associated with MEC in Adults
In a randomized, parallel, double-blind, active comparator-controlled study, fosaprepitant 150 mg as a single intravenous infusion (N=502) in combination with ondansetron and dexamethasone (intravenous fosaprepitant regimen) was compared with ondansetron and dexamethasone alone (standard therapy) (N=498) (see Table 13) in patients receiving a MEC regimen. Patient demographics were similar between the two treatment groups. Of the total 1,000 patients included in the efficacy analysis, 41% were men, 84% White, 4% Asian, 1% American Indian/Alaska Native, 2% Black, 10% Multi-Racial, and 19% Hispanic/Latino ethnicity. Patient ages ranged from 23 to 88 years of age, with a mean age of 60 years. The most commonly administered MEC chemotherapeutic agents were carboplatin (51%), oxaliplatin (24%), and cyclophosphamide (12%).
Table 13 Treatment Regimens in Adult MEC Trial* Day 1
Day 2
Day 3
Intravenous Fosaprepitant Regimen
Fosaprepitant
150 mg intravenously over 20 to 30 minutes approximately 30 minutes prior to chemotherapy
none
none
Oral Dexamethasone†
12 mg
none
none
Oral Ondansetron‡
8 mg for 2 doses
none
none
Standard Therapy
Oral Dexamethasone
20 mg
none
none
Oral Ondansetron‡
8 mg for 2 doses
8 mg twice daily
8 mg twice daily
* Fosaprepitant placebo and dexamethasone placebo (on Day 1) were used to maintain blinding.
†Dexamethasone was administered 30 minutes prior to chemotherapy treatment on Day 1. The 12 mg dose reflects a dosage adjustment to account for a drug interaction with the fosaprepitant regimen [see Clinical Pharmacology (12.3)].
‡The first ondansetron dose was administered 30 to 60 minutes prior to chemotherapy treatment on Day 1 and the second dose was administered 8 hours after first ondansetron dose.
The primary endpoint was complete response (defined as no vomiting and no rescue therapy) in the delayed phase (25 to 120 hours) of chemotherapy-induced nausea and vomiting. The results by treatment group are shown in Table 14.
Table 14 Percent of Adult Patients Receiving MEC Responding by Treatment Group ENDPOINTS
Intravenous Fosaprepitant Regimen
Standard Therapy Regimen
P-Value
Treatment Difference (95% CI)
(N=502)*
(N=498)*
%
%
PRIMARY ENDPOINT
Complete Response†
Delayed phase‡
78.9
68.5
<0.001
10.4 (5.1, 15.9)
*N: Number of patients included in the intention to treat population.
†Complete Response = no vomiting and no use of rescue therapy.
‡Delayed phase = 25 to 120 hours post-initiation of chemotherapy.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Fosaprepitant for Injection is available as follows:
One single-dose glass vial containing 150 mg fosaprepitant as a sterile, white to off-white lyophilized powder for reconstitution. Supplied as follows:
NDC 0591-4385-79 1 vial per carton.
Storage
Store at 2° to 8°C (36° to 46°F).
For storage of reconstituted drug solution see Dosage and Administration 2.2.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity
Advise patients that hypersensitivity reactions, including anaphylaxis and anaphylactic shock, have been reported in patients taking fosaprepitant. Advise patients to seek immediate medical attention if they experience signs or symptoms of a hypersensitivity reaction, such as hives, rash and itching, skin peeling or sores, flushing, difficulty in breathing or swallowing, or dizziness, rapid or weak heartbeat or feeling faint [see Warnings and Precautions (5.2)].
Infusion Site Reactions
Advise patients to seek medical attention if they experience new or worsening signs or symptoms of an infusion site reaction, such as erythema, edema, pain, necrosis, vasculitis, or thrombophlebitis at or near the infusion site [see Warnings and Precautions (5.3)].
Drug Interactions
Advise patients to discuss all medications they are taking, including other prescription, non-prescription medication or herbal products [see Contraindications (4), Warnings and Precautions (5.1)].
Warfarin: Instruct patients on chronic warfarin therapy to follow instructions from their healthcare provider regarding blood draws to monitor their INR during the 2-week period, particularly at 7 to 10 days, following initiation of Fosaprepitant for Injection with each chemotherapy cycle [see Warnings and Precautions (5.4)].
Hormonal Contraceptives: Advise patients that administration of Fosaprepitant for Injection may reduce the efficacy of hormonal contraceptives. Instruct patients to use effective alternative or back-up methods of contraception (such as condoms or spermicides) during treatment with Fosaprepitant for Injection and for 1 month following administration of Fosaprepitant for Injection [see Warnings and Precautions (5.5), Use in Specific Populations (8.3)].
Manufactured in Romania By:
Sindan Pharma SRL
11 Ion Mihalache Blvd.
Bucharest 1, Romania 011171Manufactured For:
Teva Pharmaceuticals
Parsippany, NJ 07054 -
PATIENT PACKAGE INSERT
PATIENT INFORMATION
FOSAPREPITANT for injection (FOS a PRE pi tant)
for intravenous useWhat is Fosaprepitant for Injection?
Fosaprepitant for Injection is a prescription medicine used with other medicines that treat nausea and vomiting in adults to prevent nausea and vomiting caused by certain anti-cancer (chemotherapy) medicines.
- Fosaprepitant for Injection is not used to treat nausea and vomiting that you already have.
- It is not known if Fosaprepitant for Injection is safe and effective in children.
Do not receive Fosaprepitant for Injection if you:
- are allergic to fosaprepitant, aprepitant, or any of the ingredients in Fosaprepitant for Injection. See the end of this leaflet for a complete list of the ingredients in Fosaprepitant for Injection.
- are taking pimozide (ORAP).
Before receiving Fosaprepitant for Injection, tell your healthcare provider about all your medical conditions including, if you:
- have liver problems.
- are pregnant or plan to become pregnant. It is not known if Fosaprepitant for Injection can harm your unborn baby.
- Women who use birth control medicines containing hormones to prevent pregnancy (birth control pills, skin patches, implants, and certain IUDs) should also use a backup method of birth control that does not contain hormones, such as condoms or spermicides, during treatment with Fosaprepitant for Injection and for 1 month after receiving the last dose of Fosaprepitant for Injection.
- are breastfeeding or plan to breastfeed. It is not known if Fosaprepitant for Injection passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you receive Fosaprepitant for Injection.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Fosaprepitant for Injection may affect the way other medicines work, and other medicines may affect the way Fosaprepitant for Injection works , causing serious side effects.
Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How will I receive Fosaprepitant for Injection?
Fosaprepitant for Injection will be given on Day 1 of chemotherapy treatment. It will be given to you by intravenous (IV) infusion in your vein about 50 to 60 minutes before you start your chemotherapy treatment.
If you take the blood thinner medicine warfarin sodium (COUMADIN, JANTOVEN), your healthcare provider may do blood tests after you receive Fosaprepitant for Injection to check your blood clotting.
What are the possible side effects of Fosaprepitant for Injection?
Fosaprepitant for Injection may cause serious side effects, including:
- Serious allergic reactions. Allergic reactions can happen with Fosaprepitant for Injection and may be serious. Tell your healthcare provider or nurse right away if you have hives, rash, itching, flushing or redness of your face or skin, trouble breathing or swallowing, dizziness, a rapid or weak heartbeat, or you feel faint during or soon after you receive Fosaprepitant for Injection, as you may need emergency medical care.
- Severe skin reactions, which may include rash, skin peeling, or sores, may occur. Get medical care right away if you have signs of a severe skin reaction.
- Infusion site reactions (ISR) at or near the infusion site have happened with Fosaprepitant for Injection.
Most severe ISR have happened with a certain type of chemotherapy medicine that can burn or blister your skin (vesicant) with side effects, including pain, swelling and redness. Death of skin tissue (necrosis) has happened in some people getting this type of chemotherapy medicine. Most ISR can happen with the first, second, or third dose and some can last up to 2 weeks or longer. Tell your healthcare provider right away if you get any infusion site side effects.
The most common side effects of Fosaprepitant for Injection include:
- tiredness
- feeling weak or numb in your arms and legs
- diarrhea
- indigestion or heartburn
- low white blood cell and red blood cell counts
- urinary tract infection
- weakness
- pain in your arms and legs
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of Fosaprepitant for Injection. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Fosaprepitant for Injection.
If you would like more information about Fosaprepitant for Injection, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Fosaprepitant for Injection that is written for health professionals. For more information about Fosaprepitant for Injection call Teva at 1-888-838-2872.
What are the ingredients in Fosaprepitant for Injection?
Active ingredient: fosaprepitant dimeglumine
Inactive ingredients: edetate disodium, meglumine, povidone k12, and water for injection. Hydrochloric acid and/or meglumine may have been added for pH adjustment.
Brands listed are trademarks of their respective owners.
Manufactured in Romania By: Sindan Pharma SRL, 11th Ion Mihalache Blvd., Bucharest 1, Romania 011171
Manufactured For: Teva Pharmaceuticals, Parsippany, NJ 07054This Patient Information has been approved by the U.S. Food and Drug Administration. Rev. A 6/2022
-
PRINCIPAL DISPLAY PANEL
NDC 0591-4385-79
Fosaprepitant for Injection
150 mg/vialSterile Lyophilized Powder for Intravenous Use Only After Reconstitution and Dilution
DO NOT USE WITH SOLUTIONS CONTAINING DIVALENT CATIONS (e.g., Ca2+, Mg2+) INCLUDING LACTATED RINGER’S SOLUTION AND HARTMANN’S SOLUTION.
Each vial contains:
Fosaprepitant…………….150 mg
(equivalent to 245.3 mg of fosaprepitant dimeglumine)Rx only
Single-Dose Vial
Discard Unused Portion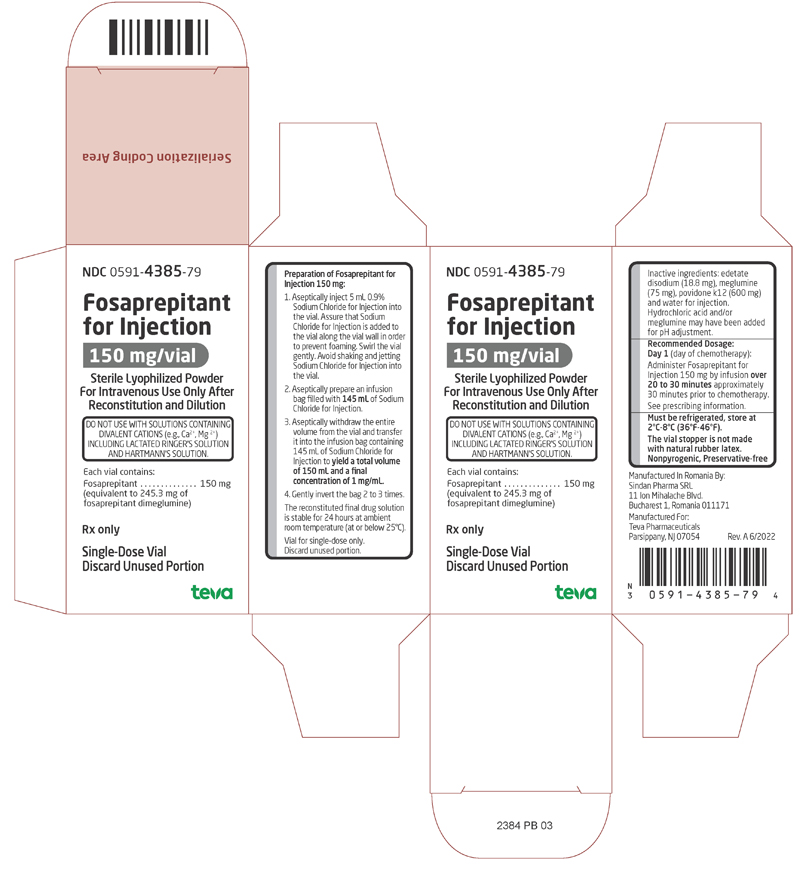
-
INGREDIENTS AND APPEARANCE
FOSAPREPITANT
fosaprepitant injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0591-4385 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOSAPREPITANT DIMEGLUMINE (UNII: D35FM8T64X) (APREPITANT - UNII:1NF15YR6UY) FOSAPREPITANT 150 mg Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) MEGLUMINE (UNII: 6HG8UB2MUY) POVIDONE K12 (UNII: 333AG72FWJ) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0591-4385-79 1 in 1 CARTON; Type 0: Not a Combination Product 09/19/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210064 09/19/2019 Labeler - Actavis Pharma, Inc. (119723554)