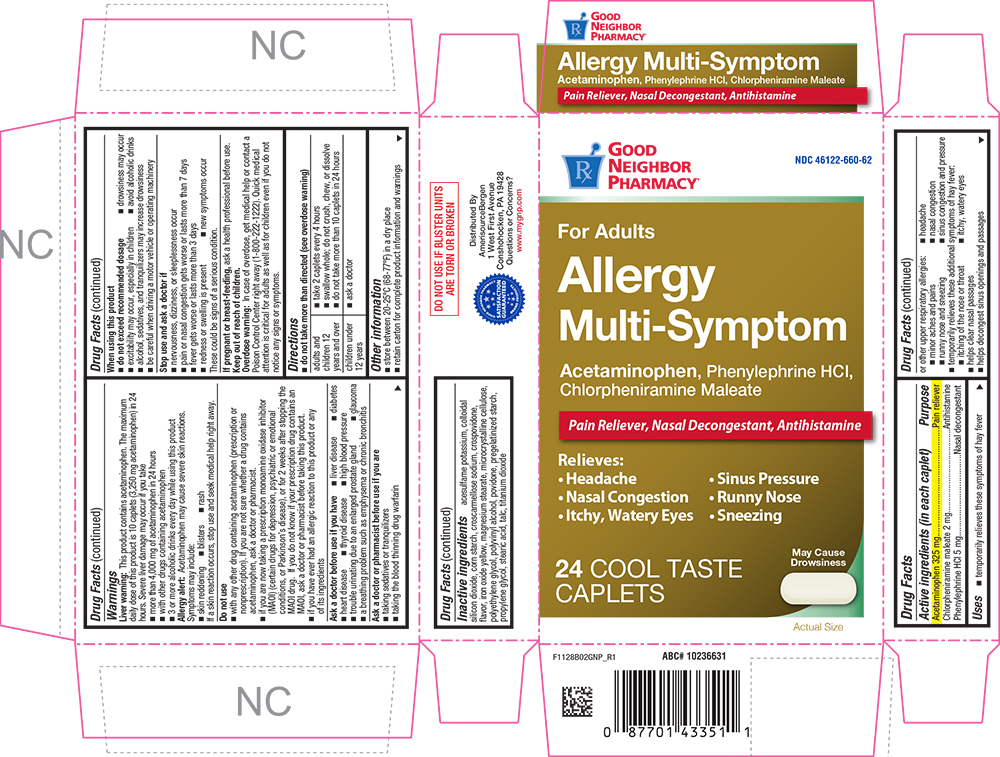
Label: ALLERGY MULTI SYMPTOM- acetaminophen, chlorpheniramine maleate, and phenylephrine hydrochloride tablet, coated
- NDC Code(s): 46122-660-62
- Packager: AMERISOURCE BERGEN
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 16, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- temporarily relieves these symptoms of hay fever or other upper respiratory allergies:
- headache
- sinus congestion and pressure
- nasal congestion
- runny nose and sneezing
- minor aches and pains
- temporarily relieves these additional symptoms of hay fever:
- itching of the nose or throat
- itchy, watery eyes
- helps clear nasal passages
- helps decongest sinus openings and passages
- temporarily relieves these symptoms of hay fever or other upper respiratory allergies:
-
Warnings
Liver warning
This product contains acetaminophen. The maximum daily dose of this product is 10 caplets (3,250 mg acetaminophen) in 24 hours. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert
Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
When using this product
- do not exceed recommended dosage
- excitability may occur, especially in children
- drowsiness may occur
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic drinks
- be careful when driving a motor vehicle or operating machinery
-
Directions
- do not take more than directed (see overdose warning)
adults and children 12 years and over - take 2 caplets every 4 hours
- swallow whole; do not crush, chew, or dissolve
- do not take more than 10 caplets in 24 hours
children under
12 years- ask a doctor
- Other information
-
Inactive ingredients
acesulfame potassium, colloidal silicon dioxide, corn starch, croscarmellose sodium, crospovidone, flavor, iron oxide yellow, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, pregelatinized starch, propylene glycol, stearic acid, talc, titanium dioxide
-
PRINCIPAL DISPLAY PANEL
GOOD NEIGHBOR PHARMACY®
NDC 46122-660-62
For Adults
Allergy Multi-Symptom
Acetaminophen, Phenylephrine HCl, Chlorpheniramine Maleate
Pain Reliever, Nasal Decongestant, Antihistamine
Relieves:
• Headache
• Snius Pressure
• Nasal Congestion
• Runny Nose
• Itchy, Watery Eyes
• Sneezing
24 COOL TASTE CAPLETS
May Cause Drowsiness
Actual Size
-
INGREDIENTS AND APPEARANCE
ALLERGY MULTI SYMPTOM
acetaminophen, chlorpheniramine maleate, and phenylephrine hydrochloride tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46122-660 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 2 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MAGNESIUM STEARATE (UNII: 70097M6I30) CROSPOVIDONE (UNII: 2S7830E561) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (Off-White) Score no score Shape OVAL Size 17mm Flavor MINT Imprint Code AAA;1128 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46122-660-62 2 in 1 CARTON 11/13/2020 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 11/13/2020 Labeler - AMERISOURCE BERGEN (007914906)