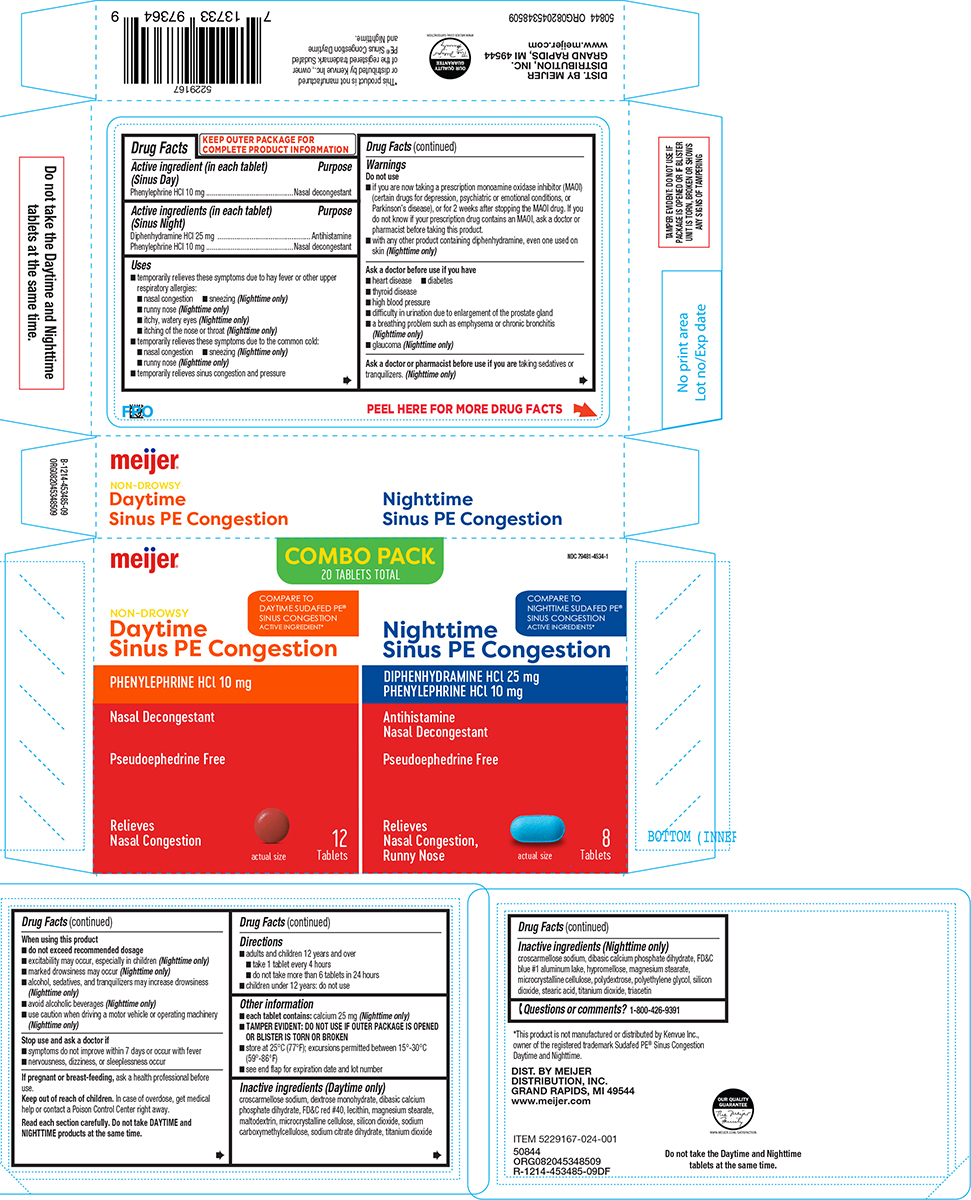
Label: SINUS PE CONGESTION DAYTIME NIGHTTIME- diphenhydramine hcl, phenylephrine hcl kit
- NDC Code(s): 79481-4534-1, 79481-7453-2, 79481-7485-3
- Packager: Meijer Distribution, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet) (Sinus Day)
- Purpose
- Active ingredients (in each tablet) (Sinus Night)
- Purpose
-
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- nasal congestion
- sneezing (Nighttime only)
- runny nose (Nighttime only)
- itchy, watery eyes (Nighttime only)
- itching of the nose or throat (Nighttime only)
- temporarily relieves these symptoms due to the common cold:
- nasal congestion
- sneezing (Nighttime only)
- runny nose (Nighttime only)
- temporarily relieves sinus congestion and pressure
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
-
Warnings
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- with any other product containing diphenhydramine, even one used on skin (Nighttime only)
Ask a doctor before use if you have
- heart disease
- diabetes
- thyroid disease
- high blood pressure
- difficulty in urination due to enlargement of the prostate gland
- a breathing problem such as emphysema or chronic bronchitis (Nighttime only)
- glaucoma (Nighttime only)
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers. (Nighttime only)
When using this product
-
do not exceed recommended dosage
- excitability may occur, especially in children (Nighttime only)
- marked drowsiness may occur (Nighttime only)
- alcohol, sedatives, and tranquilizers may increase drowsiness (Nighttime only)
- avoid alcoholic beverages (Nighttime only)
- use caution when driving a motor vehicle or operating machinery (Nighttime only)
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- Directions
- Other information
- Inactive ingredients (Daytime only)
- Inactive ingredients (Nighttime only)
- Questions or comments?
-
Principal display panel
meijer®
COMBO PACK
20 TABLETS TOTALNDC 79481-4534-1
COMPARE TO
DAYTIME SUDAFED PE®
SINUS CONGESTION ACTIVE INGREDIENT*
COMPARE TO
NIGHTTIME SUDAFED PE®
SINUS CONGESTION
ACTIVE INGREDIENTS*NON-DROWSY
Daytime
Sinus PE Congestion
PHENYLEPHRINE HCI 10 mg
NASAL DECONGESTANTPseudoephedrine Free
Relieves
Nasal Congestion12
Tablets
actual sizeNighttime
Sinus PE CongestionDIPHENHYDRAMINE HCl 25 mg
PHENYLEPHRINE HCl 10 mg
Antihistamine
Nasal DecongestantPseudoephedrine Free
Relieves
Nasal Congestion,
Runny Nose8
Tablets
actual sizeTAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERINGThis product is not manufactured
or distributed by Kenvue Inc., owner
of the registered trademark Sudafed
PE® Sinus Congestion Daytime
and Nighttime.
50844 ORG082045348509
DIST. BY MEIJER
DISTRUBUTION, INC.
GRAND RAPIDS, MI 49544
www.meijer.comDo not take the Daytime and Nighttime
tablets at the same time.
meijer 44-453485
-
INGREDIENTS AND APPEARANCE
SINUS PE CONGESTION DAYTIME NIGHTTIME
diphenhydramine hcl, phenylephrine hcl kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79481-4534 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79481-4534-1 1 in 1 CARTON; Type 0: Not a Combination Product 07/05/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 12 Part 2 1 BLISTER PACK 8 Part 1 of 2 SINUS PE CONGESTION DAYTIME
phenylephrine hcl tablet, film coatedProduct Information Item Code (Source) NDC:79481-7453 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C RED NO. 40 (UNII: WZB9127XOA) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red Score no score Shape ROUND Size 7mm Flavor Imprint Code 44;453 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79481-7453-2 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/05/2023 Part 2 of 2 SINUS PE CONGESTION NIGHTTIME
diphenhydramine hcl, phenylephrine hcl tablet, film coatedProduct Information Item Code (Source) NDC:79481-7485 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color blue Score no score Shape OVAL Size 11mm Flavor Imprint Code 44;485 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79481-7485-3 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/05/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/05/2023 Labeler - Meijer Distribution, Inc. (006959555) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(79481-4534) , pack(79481-4534) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(79481-4534) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 manufacture(79481-4534) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(79481-4534)