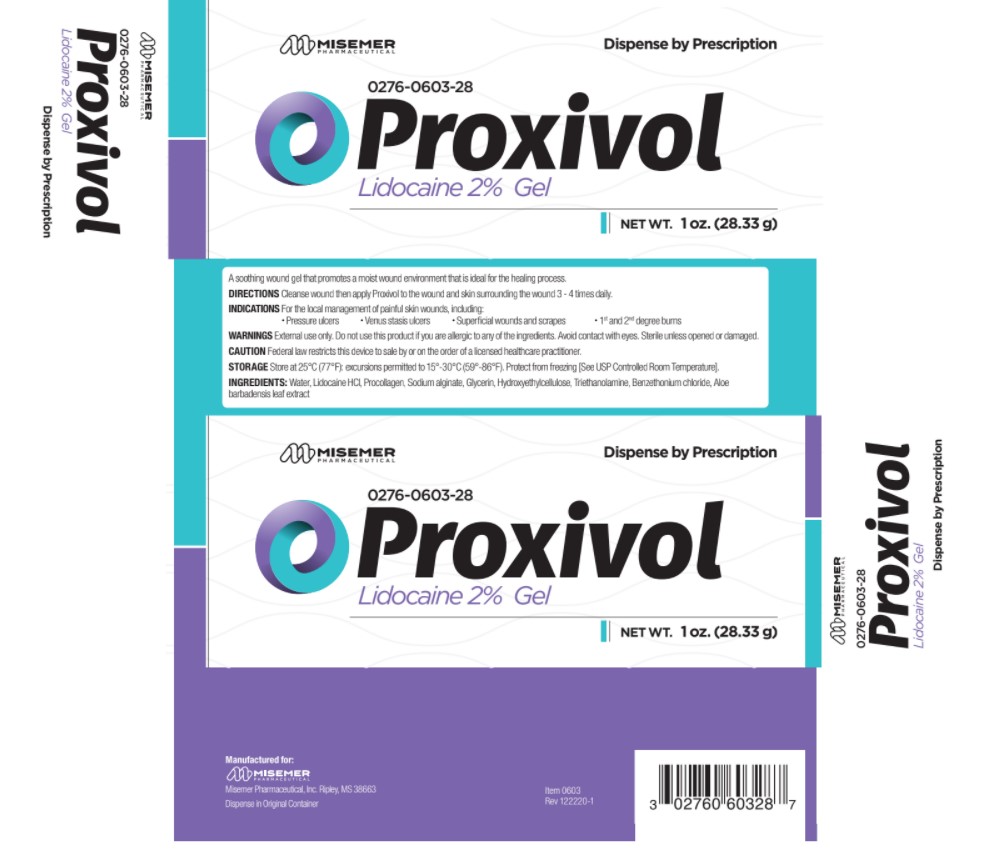
Label: PROXIVOL-
- NHRIC Code(s): 0276-0603-28
- Packager: Misemer Pharmaceutical
- Category: PRESCRIPTION MEDICAL DEVICE LABEL
- DEA Schedule: None
- Marketing Status: Premarket Notification
Drug Label Information
Updated February 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Directions:
- Indications:
- Warnings:
- Caution:
- Storage:
- Ingredients:
- CALL YOUR DOCTOR ABOUT SIDE EFFECTS:
- How Supplied:
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PROXIVOL
dressing, wound and burn, hydrogel w/drug and/or biologicProduct Information Product Type PRESCRIPTION MEDICAL DEVICE Item Code (Source) NHRIC:0276-0603 Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) BOVINE TYPE I COLLAGEN (UNII: FHJ3ATL51C) ALOE VERA LEAF (UNII: ZY81Z83H0X) SODIUM ALGINATE (UNII: C269C4G2ZQ) GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) TROLAMINE (UNII: 9O3K93S3TK) BENZETHONIUM CHLORIDE (UNII: PH41D05744) Product Characteristics (SPLSTERILEUSE) false (SPLMRISAFE) true Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:0276-0603-28 28.33 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date premarket notification K020540 02/01/2022 Labeler - Misemer Pharmaceutical (784121365)