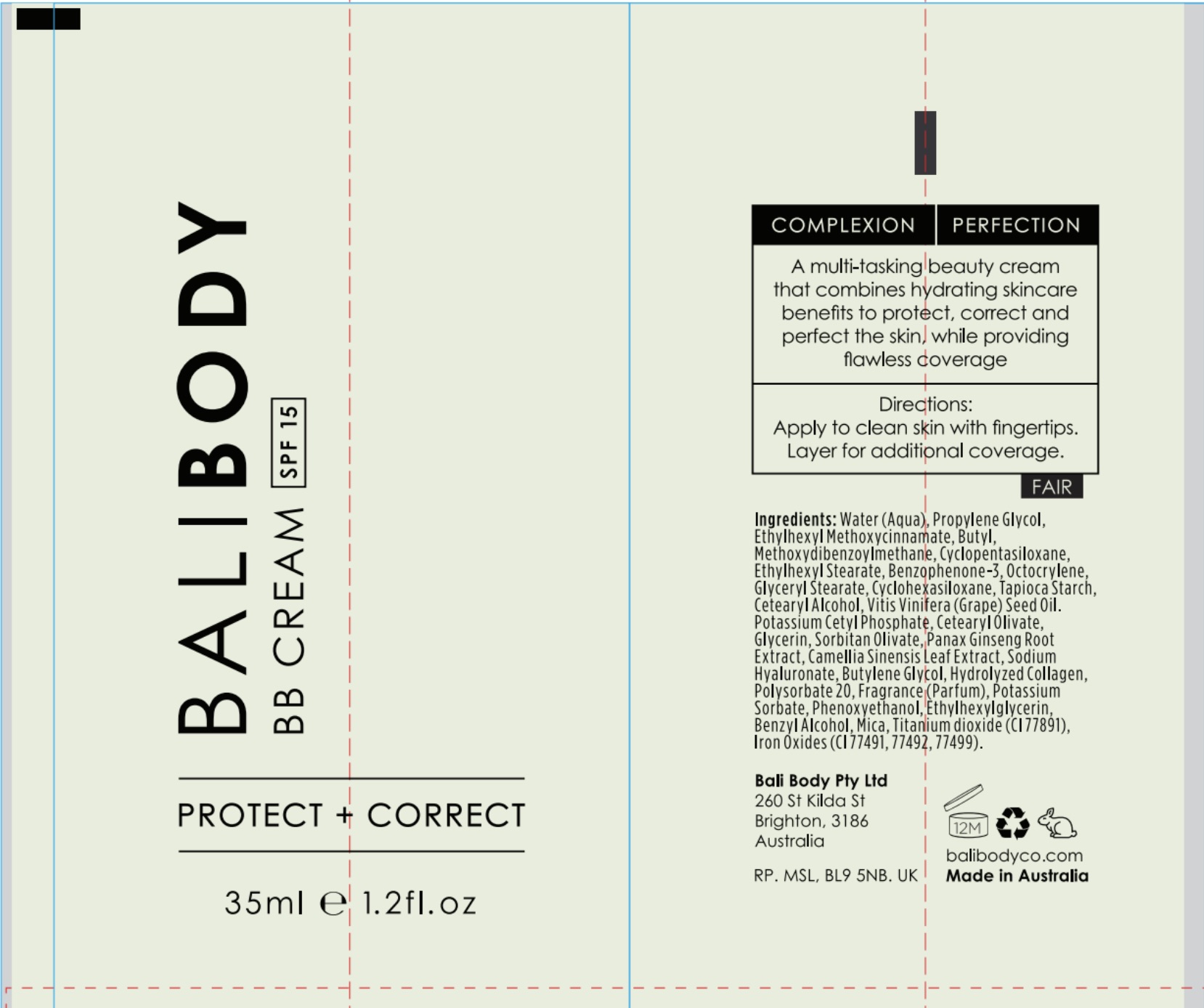
Label: BALI BODY BB CREAM SPF 15- octinoxate, octocrylene, ocybenzone, avobenzone cream cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 70630-1605-1, 70630-1605-2, 70630-1605-3 - Packager: Bali Body Pty Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 6, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL



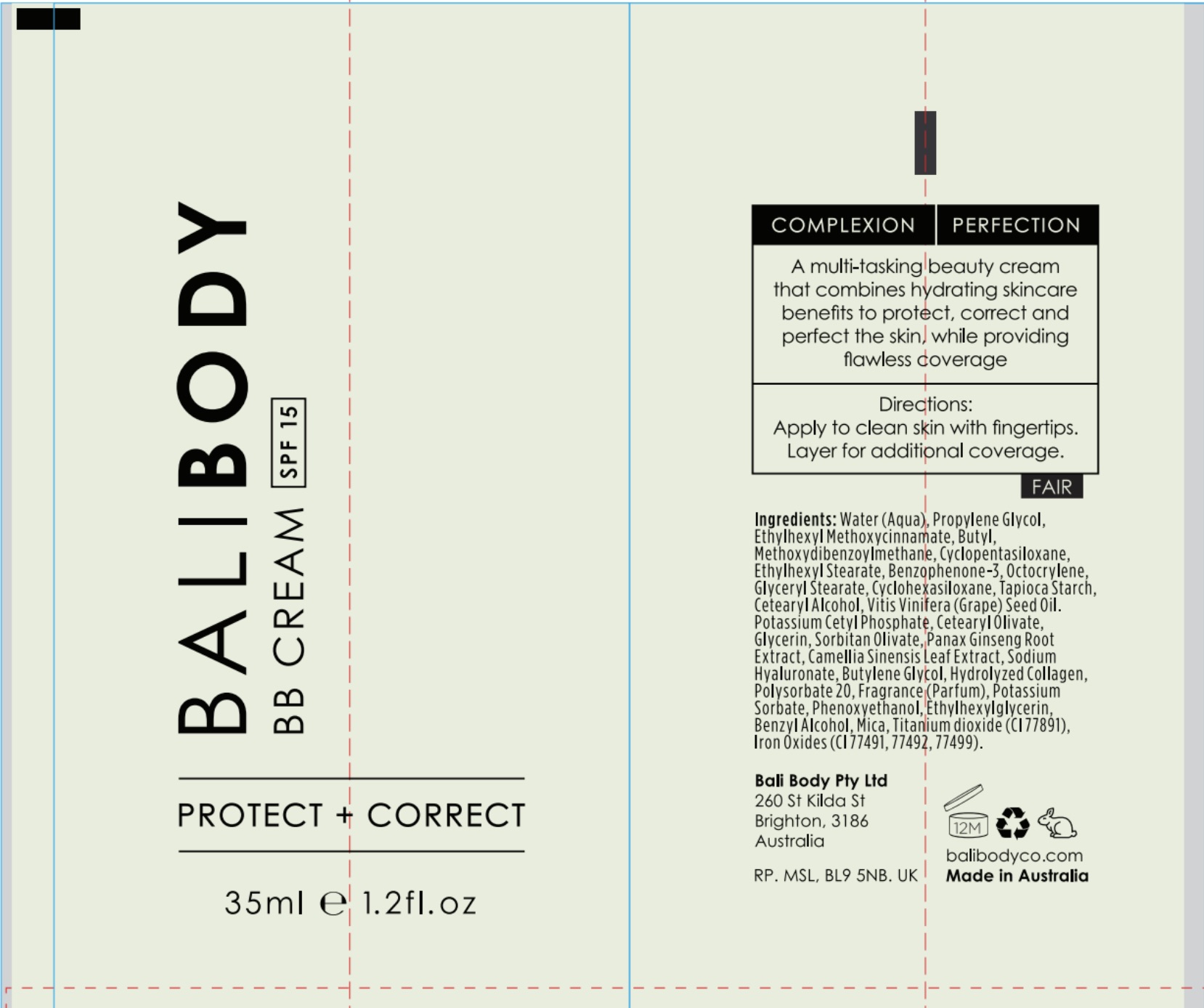
Shake well before use. Apply liberaly 15 minutes before sun exposure.
Children under 6 months old: Ask a doctor.
Re-apply every 2 hours.
Use water-resistant sunscreen if swimming or sweating.
Store in cool dry place.
Skin Cancer/Skin Ageing Alert: Spending time in the sun increases your risk of skin cancer and early skin aging.
This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
Always wear a hat, protective clothing and sunglasses.
-
INGREDIENTS AND APPEARANCE
BALI BODY BB CREAM SPF 15
octinoxate, octocrylene, ocybenzone, avobenzone cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70630-1605 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2.7 mg in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 4 mg in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 4 mg in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 3 mg in 100 mL Inactive Ingredients Ingredient Name Strength GRAPE SEED OIL (UNII: 930MLC8XGG) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) STARCH, TAPIOCA (UNII: 24SC3U704I) ASIAN GINSENG (UNII: CUQ3A77YXI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL OLIVATE (UNII: 58B69Q84JO) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HYALURONATE SODIUM (UNII: YSE9PPT4TH) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYSORBATE 20 (UNII: 7T1F30V5YH) BENZYL ALCOHOL (UNII: LKG8494WBH) GLYCERIN (UNII: PDC6A3C0OX) SORBITAN OLIVATE (UNII: MDL271E3GR) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) FERROUS OXIDE (UNII: G7036X8B5H) CYCLOMETHICONE 6 (UNII: XHK3U310BA) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70630-1605-1 35 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/08/2016 08/31/2021 2 NDC:70630-1605-2 35 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/08/2016 09/30/2022 3 NDC:70630-1605-3 3 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/08/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 06/08/2016 Labeler - Bali Body Pty Ltd (757840223) Registrant - Bali Body Pty Ltd (757840223)