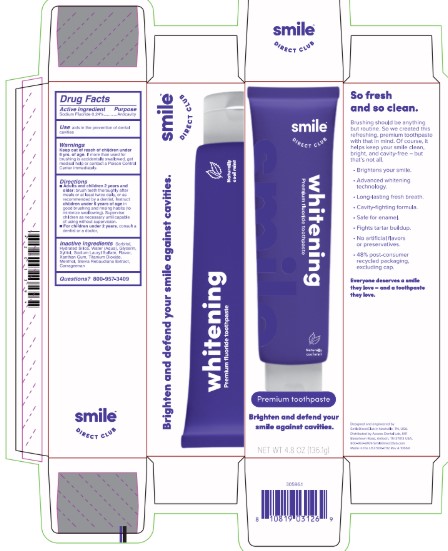
Label: SMILE DIRECT CLUB- sodium fluoride toothpaste paste, dentifrice
-
Contains inactivated NDC Code(s)
NDC Code(s): 73333-125-03 - Packager: Nutrix International, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 16, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Indications and Usage Section
- Warnings
- Keep out of Reach of Children
-
Directions
- Adults and children 2 years of age and older. Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor.
- Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing).
- Supervise children as necessary until capable of using without supervision.
- Children under 2 years of age. Consult a dentist or doctor.
- Inactive Ingredients
- Questions?
- Principle package label
- Primary Packaging - Carton
-
INGREDIENTS AND APPEARANCE
SMILE DIRECT CLUB
sodium fluoride toothpaste paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73333-125 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 2.4 mg in 1 g Inactive Ingredients Ingredient Name Strength HYDRATED SILICA (UNII: Y6O7T4G8P9) WATER (UNII: 059QF0KO0R) XYLITOL (UNII: VCQ006KQ1E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MINT (UNII: FV98Z8GITP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) STEVIA REBAUDIUNA LEAF (UNII: 6TC6NN0876) CARRAGEENAN (UNII: 5C69YCD2YJ) SORBITOL (UNII: 506T60A25R) MENTHOL (UNII: L7T10EIP3A) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color white Score Shape Size Flavor MINT (Cool Mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73333-125-03 1 in 1 CARTON 09/25/2019 1 125 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 09/25/2019 Labeler - Nutrix International, LLC (117341868) Establishment Name Address ID/FEI Business Operations Nutrix International,LLC 117341868 manufacture(73333-125)