Label: MENTHOL CAMPHOR KNEE (menthol, unspecified form and camphor- natural patch
- NDC Code(s): 71391-139-01, 71391-139-05, 71391-139-06, 71391-139-18
- Packager: Unexo Life Sciences Private Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
For external use only
When using this product
- use only as directed
- Do not bandage tightly or use with a heating pad
- Avoid contact with eyes and mucous membranes
- Do not apply to wounds or damaged, broken or irritated skin
- Do not use at the same time as other topical analgesics
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
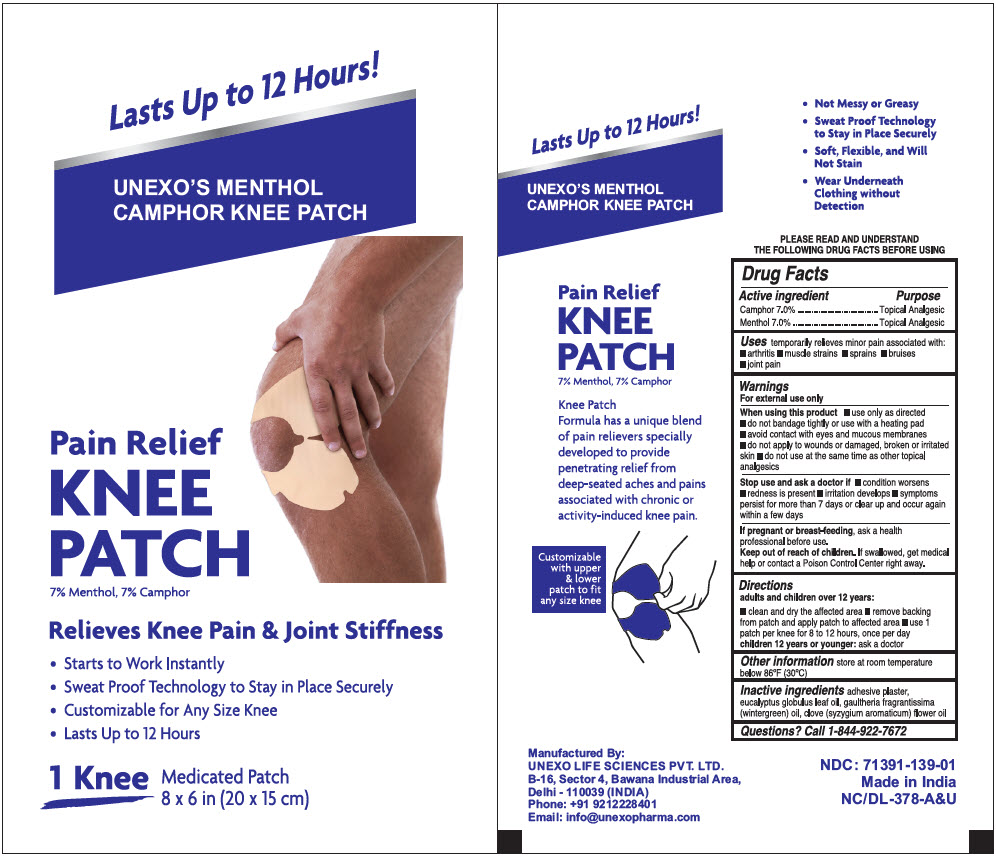
PRINCIPAL DISPLAY PANEL - 1 Patch Pouch Box
Lasts Up to 12 Hours!
UNEXO'S MENTHOL
CAMPHOR KNEE PATCHPain Relief
KNEE
PATCH
7% Menthol, 7% CamphorRelieves Knee Pain & Joint Stiffness
- Starts to Work Instantly
- Sweat Proof Technology to Stay in Place Securely
- Customizable for Any Size Knee
- Lasts Up to 12 Hours
1 Knee Medicated Patch
8 x 6 in (20 x 15 cm)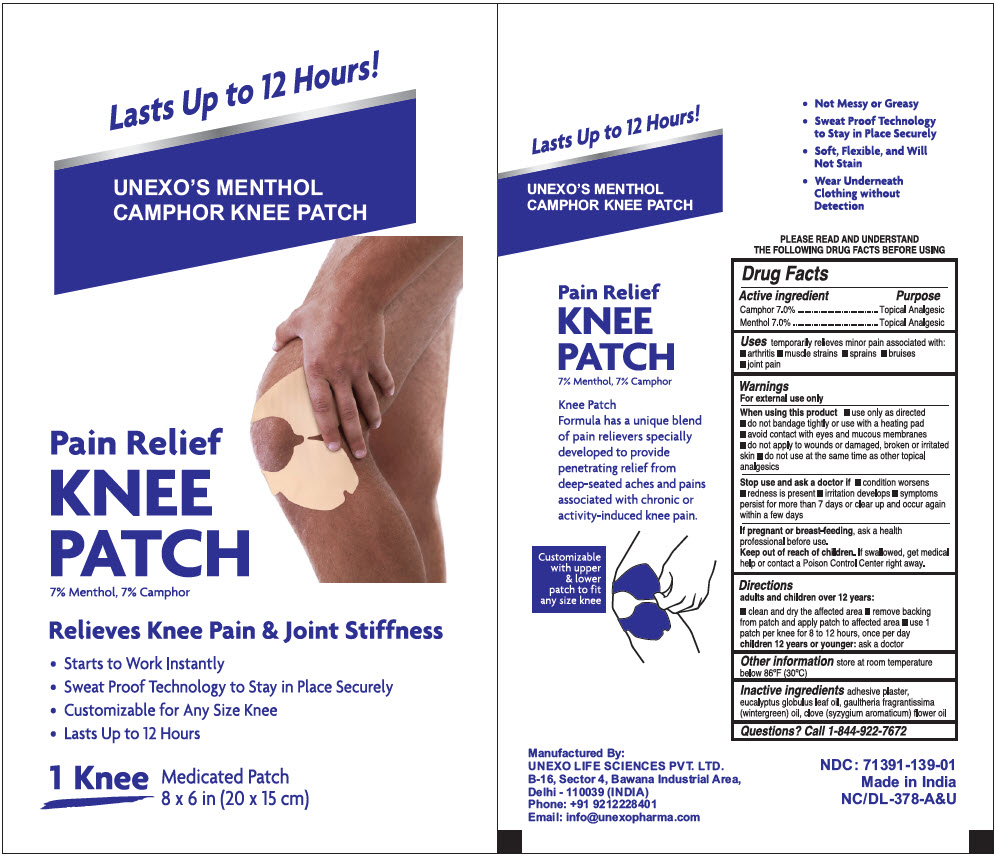
-
INGREDIENTS AND APPEARANCE
MENTHOL CAMPHOR KNEE
menthol, unspecified form and camphor (natural) patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71391-139 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 308 mg CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 308 mg Inactive Ingredients Ingredient Name Strength Eucalyptus oil (UNII: 2R04ONI662) METHYL SALICYLATE (UNII: LAV5U5022Y) Clove Oil (UNII: 578389D6D0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71391-139-06 6 in 1 BOX 12/22/2023 1 NDC:71391-139-01 1 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:71391-139-18 18 in 1 BOX 12/22/2023 2 NDC:71391-139-01 1 in 1 POUCH; Type 0: Not a Combination Product 3 NDC:71391-139-05 5 in 1 BOX 12/22/2023 3 NDC:71391-139-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M017 12/22/2023 Labeler - Unexo Life Sciences Private Limited (872260479) Establishment Name Address ID/FEI Business Operations Unexo Life Sciences Private Limited 872260479 MANUFACTURE(71391-139)