Label: FEXOFENADINE HCL tablet
- NDC Code(s): 16714-899-01, 16714-899-02
- Packager: NorthStar RxLLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 30, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
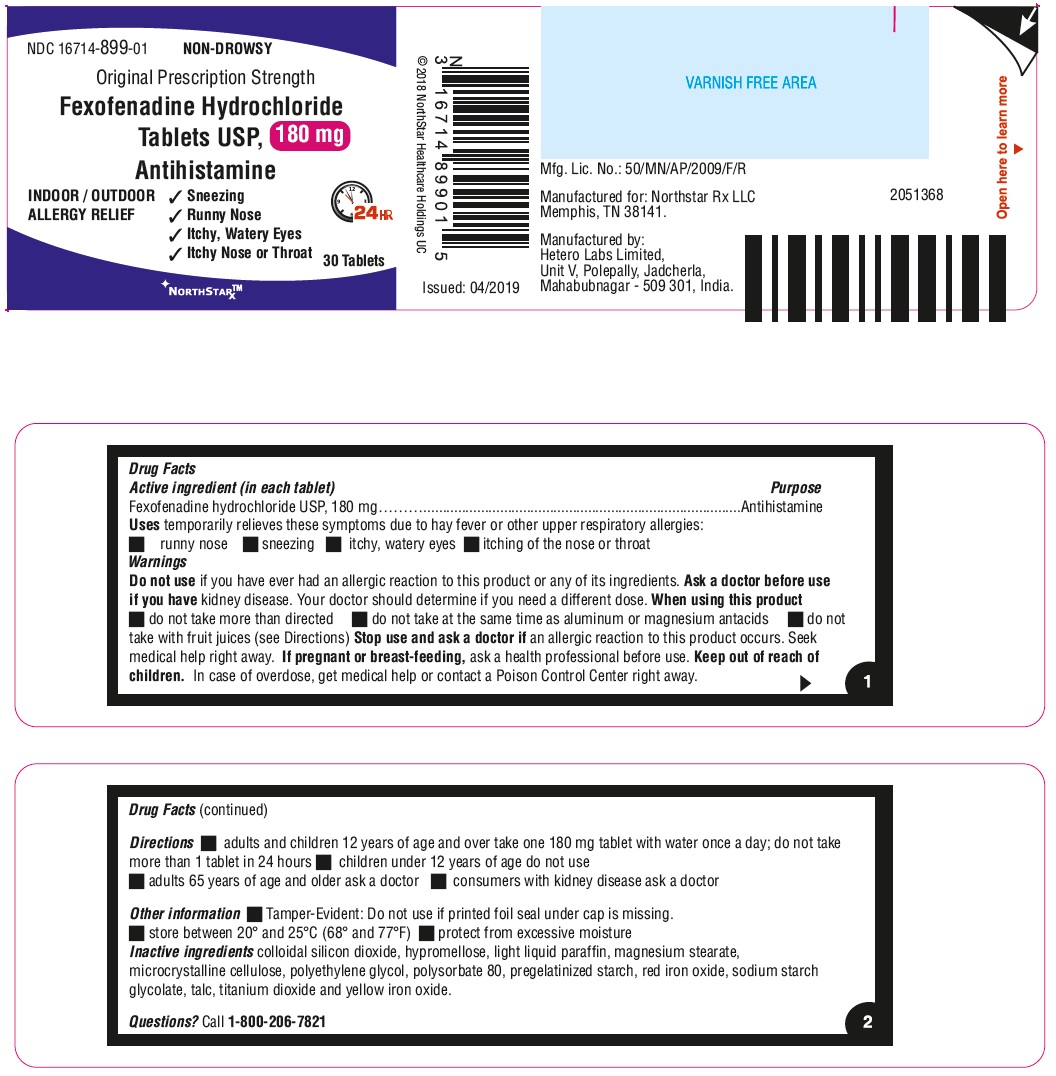
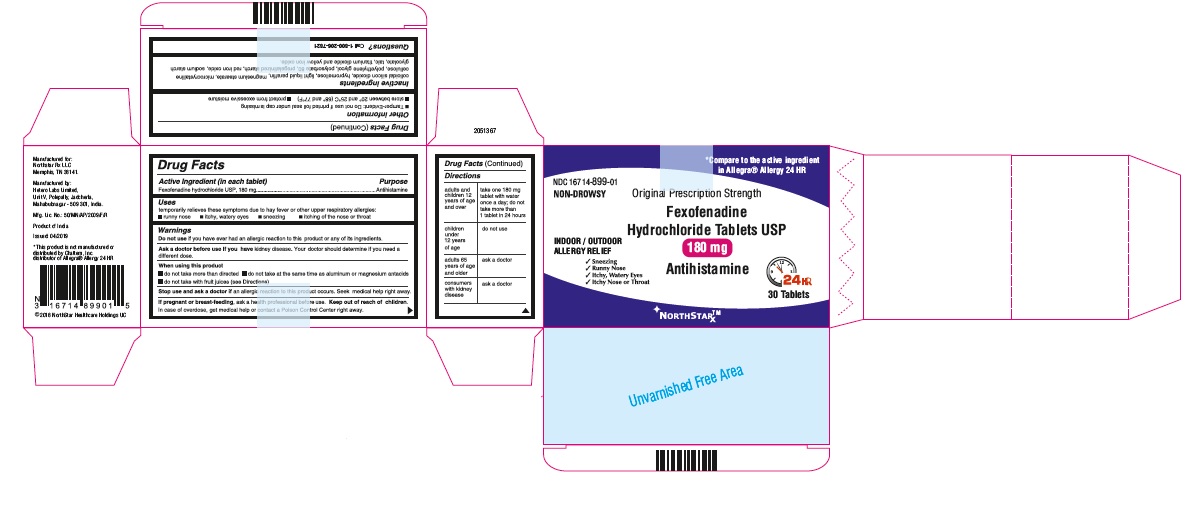
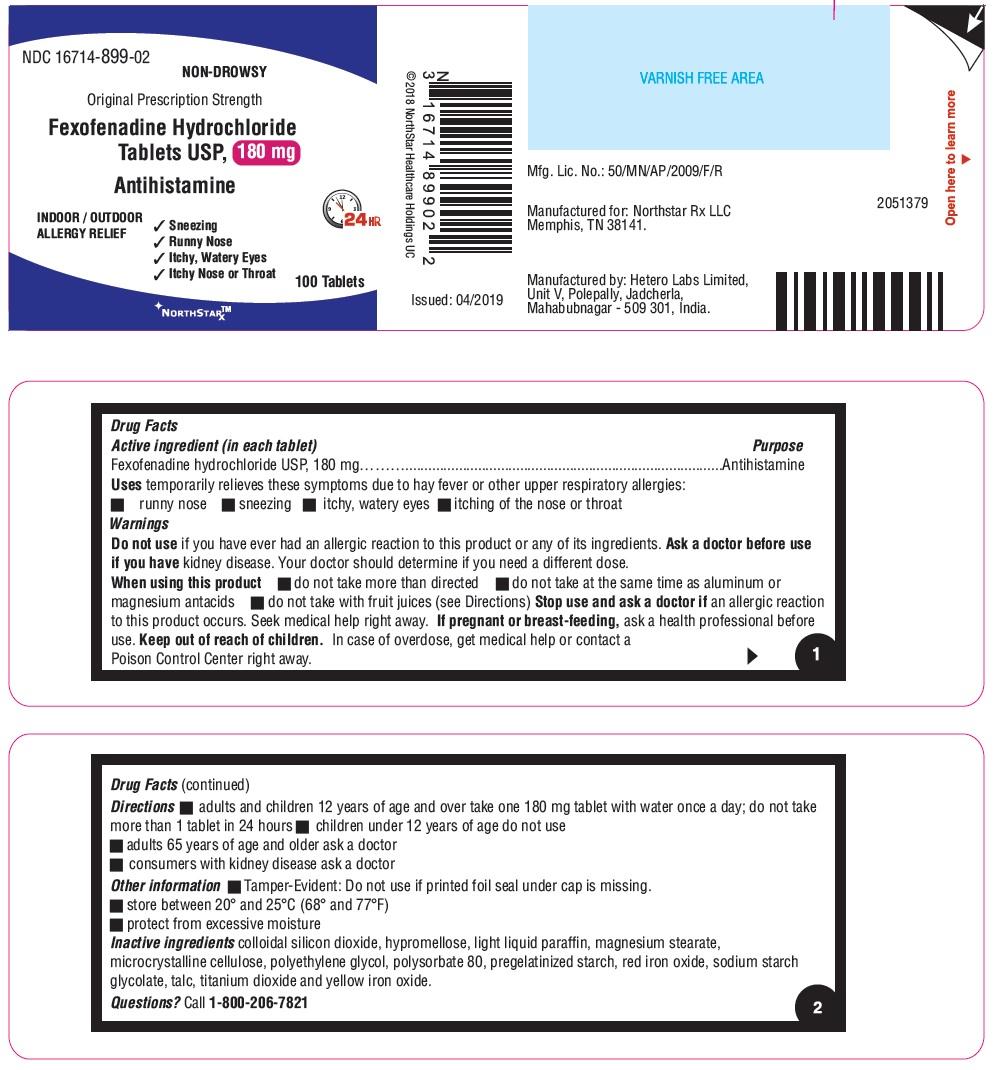
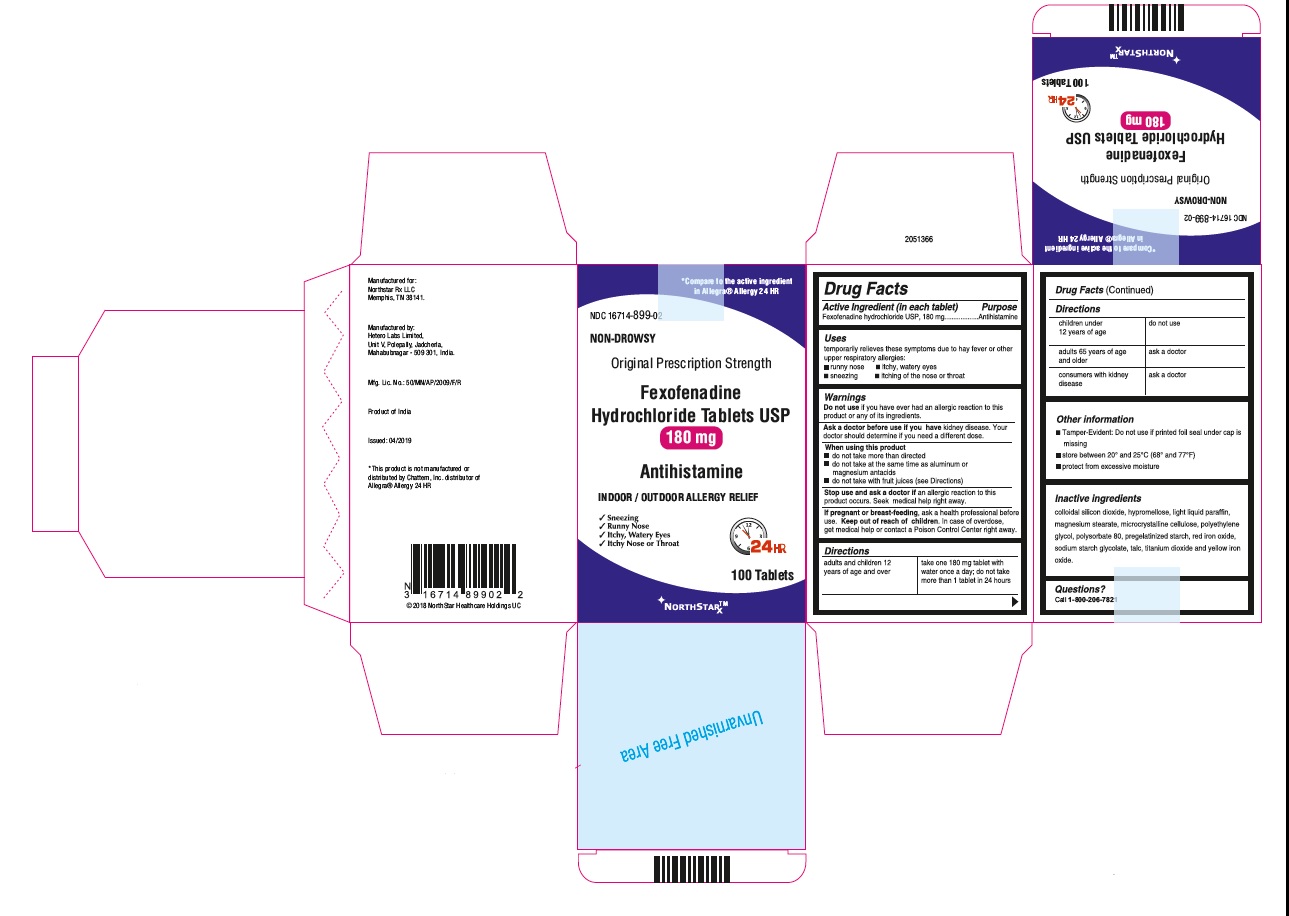
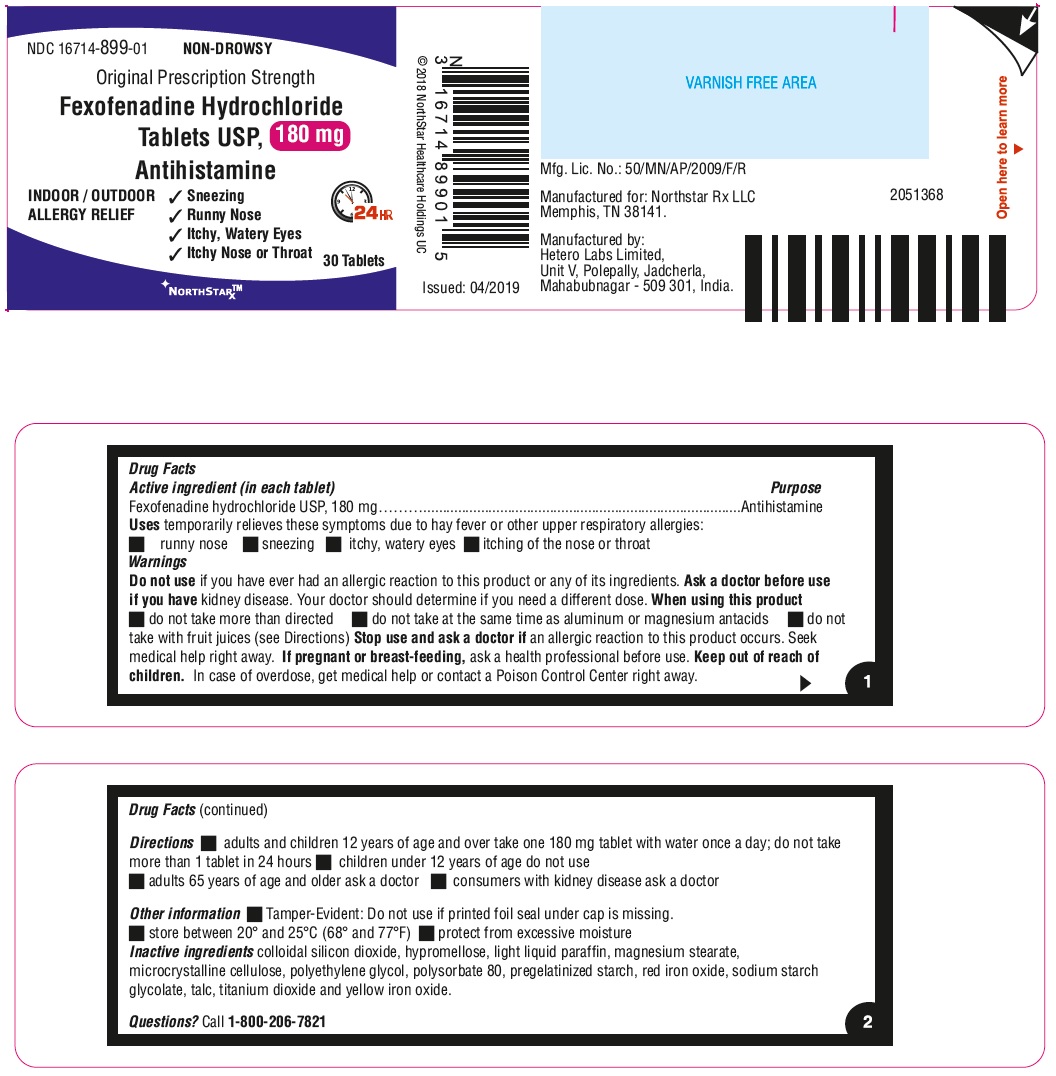
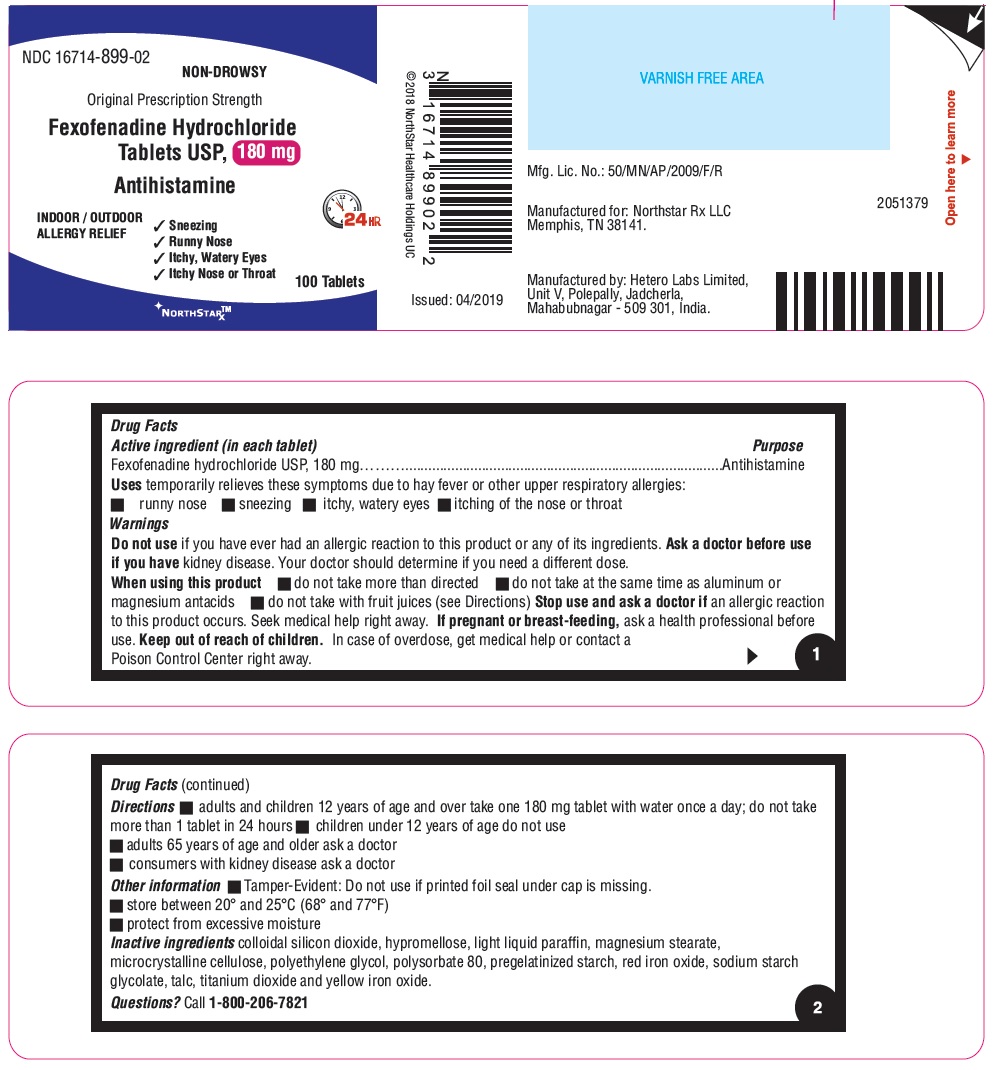
- ACTIVE INGREDIENT(S) in each tablet
- PURPOSE
- USE(S)
- WARNINGS
- DO NOT USE
- ASK A DOCTOR BEFORE USE IF
- WHEN USING THIS PRODUCT
- STOP USE AND ASK A DOCTOR IF
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- Other information
- Inactive ingredients
- Questions?
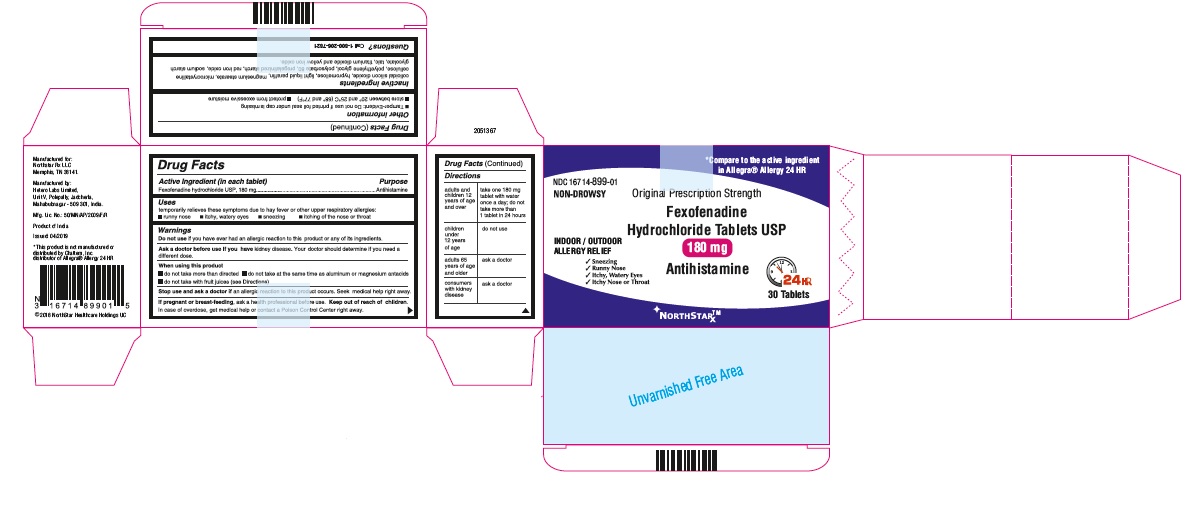
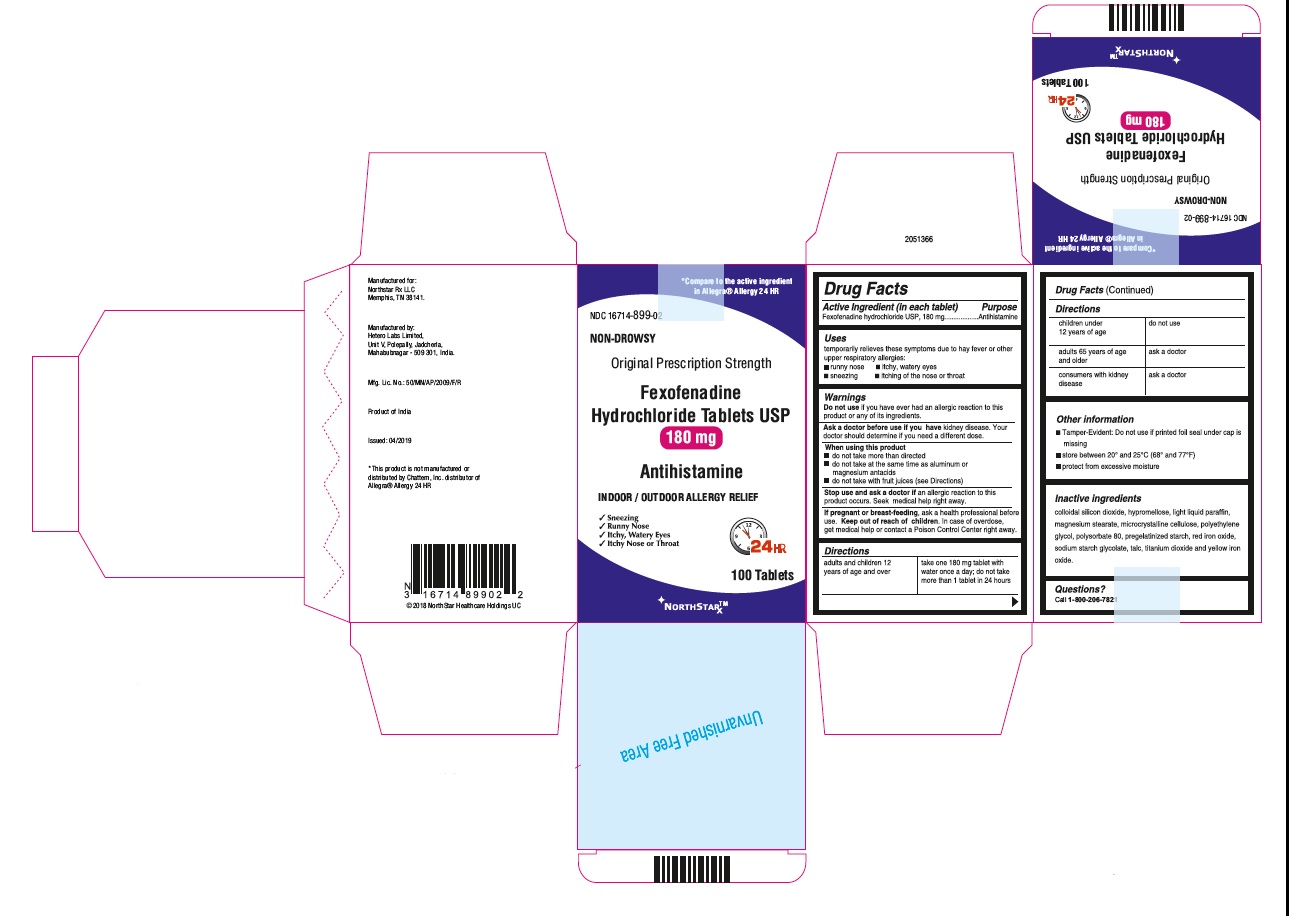
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HCL
fexofenadine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:16714-899 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LIGHT MINERAL OIL (UNII: N6K5787QVP) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) POLYSORBATE 80 (UNII: 6OZP39ZG8H) STARCH, CORN (UNII: O8232NY3SJ) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PINK Score no score Shape CAPSULE Size 18mm Flavor Imprint Code J;44 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16714-899-01 1 in 1 CARTON 02/18/2019 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:16714-899-02 1 in 1 CARTON 02/18/2019 2 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204097 02/18/2019 Labeler - NorthStar RxLLC (830546433) Establishment Name Address ID/FEI Business Operations Hetero Labs Limited Unit V 650452530 ANALYSIS(16714-899) , MANUFACTURE(16714-899)