Label: ARAMARK EXTRA STRENGTH ACETAMINOPHEN- acetaminophen tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 81238-3500-1, 81238-3500-2 - Packager: Western First Aid Safety DBA Aramark
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 19, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings:
Allergy Alert: Acetaminophen may cause a severe skin
reactions.
Symptoms may include:
• Skin reddening •Blisters •Rash
• If a skin reaction occurs, stop use and seek medical
right away.Liver Warning: This product contains an
acetaminophen. Severe liver damage may occur if you
take:
• more than 8 tablets in 24 hours, which is the
maximum daily amount
• 3 or more alcoholic drinks every day while using this
product• take more or for a longer time than directed
- DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
-
Package Labeling
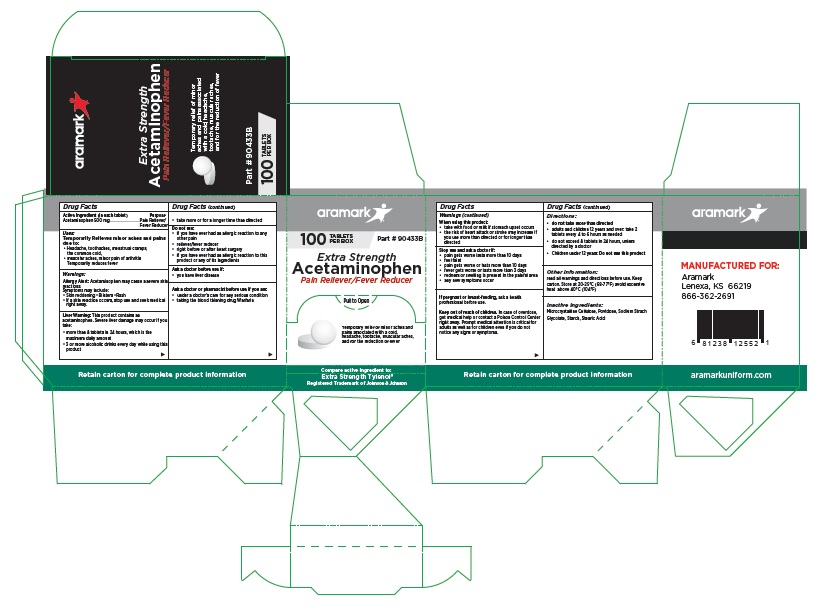
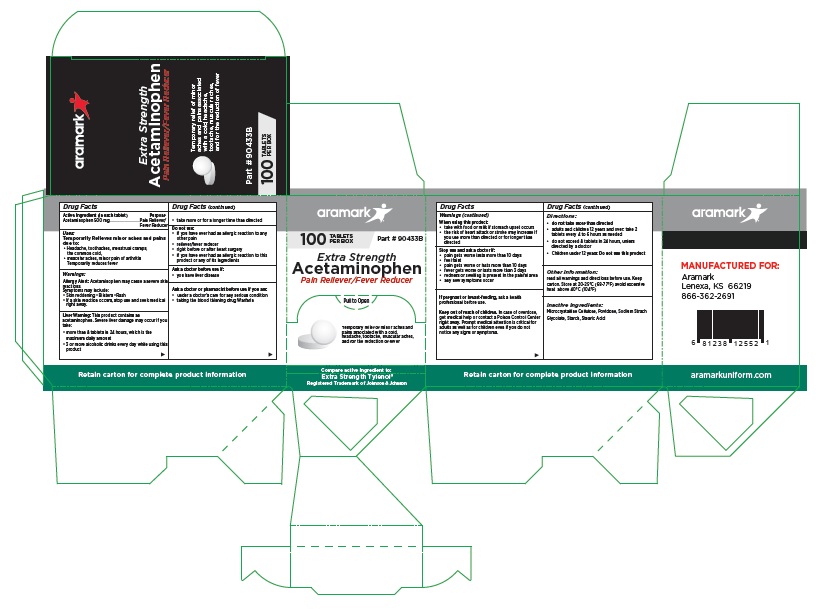
aramark
100 TABLETS Part # 90433B
PER BOXExtra Strength
Acetaminophen
Pain Reliever/Fever ReducerTemporary relief of minor aches and
pains associated with a cold,
headache, tootache, muscular aches,
and for the reduction of feverCompare active ingredient to:
Extra Strength Tylenol®
Registered Trademark of Johnson & JohnsonMANUFACTURED FOR:
Aramark
Lenexa, KS 66219
866-362-2691aramarkuniform.com
Retain carton for complete product information
100 Tablet Box
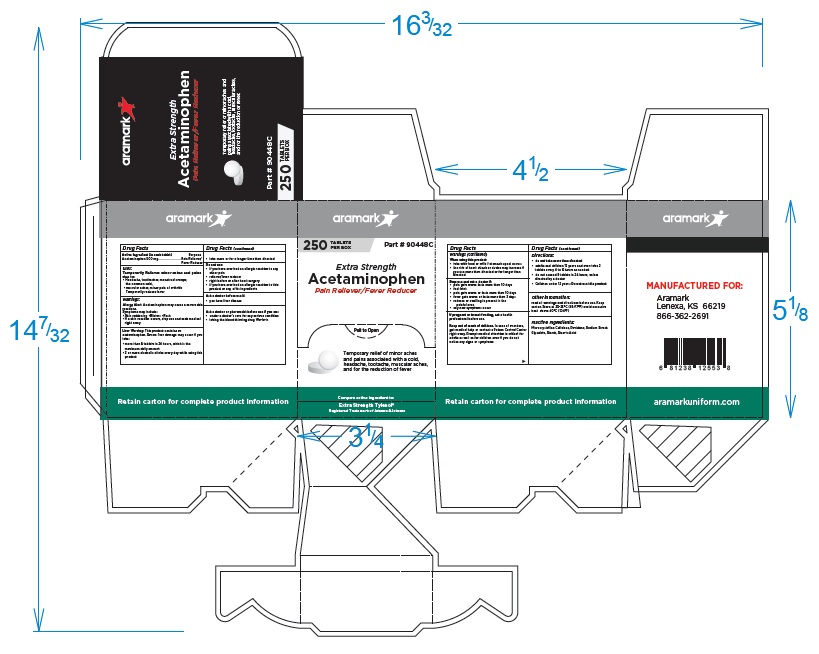
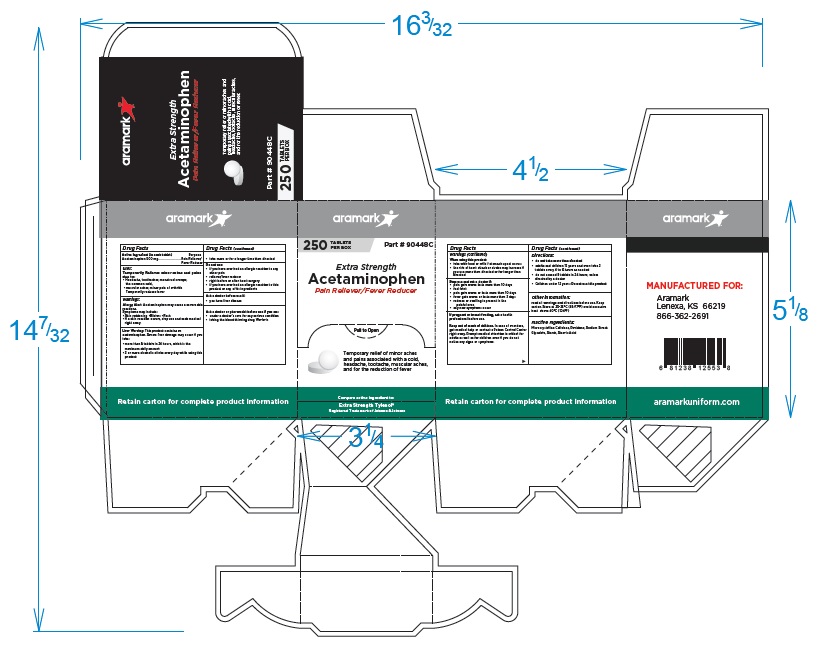
250 Tablet Box
2-Tablet Packet

res
-
INGREDIENTS AND APPEARANCE
ARAMARK EXTRA STRENGTH ACETAMINOPHEN
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81238-3500 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score no score Shape ROUND Size 18mm Flavor Imprint Code FR1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81238-3500-1 50 in 1 BOX 05/14/2021 1 2 in 1 PACKET; Type 0: Not a Combination Product 2 NDC:81238-3500-2 125 in 1 BOX 05/14/2021 2 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 05/14/2021 Labeler - Western First Aid Safety DBA Aramark (043861524) Registrant - Western First Aid Safety DBA Aramark (043861524) Establishment Name Address ID/FEI Business Operations ULTRA SEAL CORPORATION 085752004 pack(81238-3500) Establishment Name Address ID/FEI Business Operations ULTRA SEAL CORPORATION 944090448 manufacture(81238-3500)