Label: EVZIO- R NALOXONE HCL injection, solution
- NDC Code(s): 51662-1495-1
- Packager: HF Acquisition Co LLC, DBA HealthFirst
- This is a repackaged label.
- Source NDC Code(s): 60842-051
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated February 23, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EVZIO® safely and effectively. See full prescribing information for EVZIO.
EVZIO® (naloxone hydrochloride injection) Auto-Injector for intramuscular or subcutaneous use
2 mg
Initial U.S. Approval: 1971INDICATIONS AND USAGE
EVZIO is an opioid antagonist indicated for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression in adults and pediatric patients. ( 1)
EVZIO is intended for immediate administration as emergency therapy in settings where opioids may be present. ( 1)
EVZIO is not a substitute for emergency medical care. ( 1)
DOSAGE AND ADMINISTRATION
EVZIO is for intramuscular or subcutaneous use only. ( 2-2.1)
Seek emergency medical care immediately after use. ( 2-2.1)
Administer EVZIO to adult or pediatric patients into the anterolateral aspect of the thigh, through clothing if necessary. ( 2-2.2)
Administer additional doses of EVZIO, using a new auto-injector, if the patient does not respond or responds and then relapses into respiratory depression. Additional doses of EVZIO may be given every 2 to 3 minutes until emergency medical assistance arrives. ( 2-2.2)
Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance. ( 2-2.2)
In pediatric patients under the age of one, the caregiver should pinch the thigh muscle while administering the dose. ( 2-2.2)
If the electronic voice instruction system does not operate properly, EVZIO will still deliver the intended dose of naloxone hydrochloride when used according to the printed instructions on the flat surface of its label. ( 2-2.1)DOSAGE FORMS AND STRENGTHS
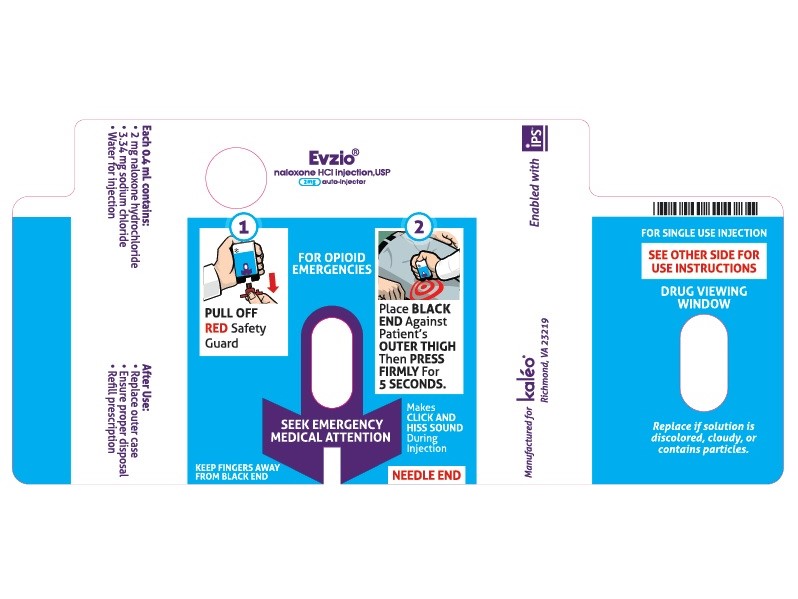
Injection: 2 mg/0.4 mL naloxone hydrochloride solution in a pre-filled auto-injector. ( 3
CONTRAINDICATIONS
Hypersensitivity to naloxone hydrochloride. ( 4)
WARNINGS AND PRECAUTIONS
Risk of Recurrent Respiratory and CNS Depression: Due to the duration of action of naloxone relative to the opioid, keep the patient under continued surveillance and administer repeated doses of naloxone using a new EVZIO, as necessary, while awaiting emergency medical assistance. ( 5-5.1)
Risk of Limited Efficacy with Partial Agonists or Mixed Agonists/Antagonists: Reversal of respiratory depression caused by partial agonists or mixed agonists/antagonists such as buprenorphine and pentazocine, may be incomplete. Larger or repeat doses may be required. ( 5-5.2)
Precipitation of Severe Opioid Withdrawal: Use in patients who are opioid dependent may precipitate opioid withdrawal. In neonates, opioid withdrawal may be life-threatening if not recognized and properly treated. Monitor for the development of opioid withdrawal. ( 5-5.3)
Risk of Cardiovascular (CV) Effects: Abrupt postoperative reversal of opioid depression may results in adverse CV effects. These events have primarily occurred in patients who had pre-existing CV disorders or received other drugs that may have similar CV effects. Monitor these patients closely in an appropriate healthcare setting after use of naloxone hydrochloride. ( 5-5.3)ADVERSE REACTIONS
The following adverse reactions were most commonly observed in EVZIO clinical studies: dizziness and injection site erythema. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact contact kaleo, Inc. at 1-855-773-8946 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2016
-
TABLE OF CONTENTS
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosing Information
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Recurrent Respiratory and Central Nervous System Depression
5.2 Risk of Limited Efficacy with Partial Agonists or Mixed Agonist/Antagonists
5.3 Precipitation of Severe Opioid Withdrawal
5 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION*
Sections or subsections omitted from the full prescribing information are not listed. -
1 INDICATIONS & USAGE
EVZIO is an opioid antagonist indicated for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression in adults and pediatric patients.
EVZIO is intended for immediate administration as emergency therapy in settings where opioids may be present.
EVZIO is not a substitute for emergency medical care.
-
2 DOSAGE & ADMINISTRATION
2.1 Important Administration Instructions
EVZIO is for intramuscular and subcutaneous use only.
Because treatment of suspected opioid overdose must be performed by someone other than the patient, instruct the prescription recipient to inform those around them about the presence of EVZIO and the Instructions for Use.
Instruct the patient or caregiver to read the Instructions for Use at the time they receive a prescription for EVZIO. Emphasize the following instructions to the patient or caregiver:
Seek emergency medical care immediately after use. Since the duration of action of most opioids exceeds that of naloxone hydrochloride and the suspected opioid overdose may occur outside of supervised medical settings, seek immediate emergency medical assistance, keep the patient under continued surveillance until emergency personnel arrive, and administer repeated doses of EVZIO as necessary. Always seek emergency medical assistance in the event of a suspected potentially life-threatening opioid emergency after administration of the first dose of EVZIO.
Additional doses of EVZIO may be required until emergency medical assistance becomes available.
Do not attempt to reuse EVZIO. Each EVZIO contains a single dose of naloxone.
Visually inspect EVZIO through the viewing window for particulate matter and discoloration prior to administration. Do not administer unless the solution is clear and the glass container is undamaged.
EVZIO must be administered according to the printed instructions on the device label or the electronic voice instructions (EVZIO contains a speaker that provides voice instructions to guide the user through each step of the injection). If the EVZIO electronic voice instruction system does not operate properly, EVZIO will still deliver the intended dose of naloxone hydrochloride when used according to the printed instructions on its label.
Once the red safety guard is removed, EVZIO must be used immediately or disposed of properly. Do not attempt to replace the red safety guard once it is removed.Upon actuation, EVZIO automatically inserts the needle intramuscularly or subcutaneously, delivers the naloxone hydrochloride injection, and retracts the needle fully into its housing. Post injection, the black base locks in place, a red indicator appears in the viewing window, and electronic visual and audible instructions signal that EVZIO has delivered the intended dose of naloxone hydrochloride and instructs the user to seek emergency medical attention.
2.2 Dosing Information
Initial Dosing
Administer the initial dose of EVZIO to adult or pediatric patients intramuscularly or subcutaneously into the anterolateral aspect of the thigh, through clothing if necessary, and seek emergency medical assistance. Administer EVZIO as quickly as possible because prolonged respiratory depression may result in damage to the central nervous system or death.
Repeat Dosing
The requirement for repeat doses of EVZIO depends upon the amount, type, and route of administration of the opioid being antagonized.
If the desired response is not obtained after 2 or 3 minutes, an additional dose of EVZIO may be administered. If there is still no response and additional doses are available, additional doses of EVZIO may be administered every 2 to 3 minutes until emergency medical assistance arrives. Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance.
If the patient responds to EVZIO and relapses back into respiratory depression before emergency assistance arrives, an additional dose of EVZIO may be administered.
Reversal of respiratory depression by partial agonists or mixed agonist/antagonists, such as buprenorphine and pentazocine, may be incomplete and may require higher doses of naloxone hydrochloride or repeated administration of EVZIO.
Dosing in Adults and Pediatric Patients over Age One Year
Instruct patients or their caregivers to administer EVZIO according to the Instructions for Use, intramuscularly or subcutaneously.
Dosing in Pediatric Patients under Age One Year
In pediatric patients under the age of one year, the caregiver should pinch the thigh muscle while administering EVZIO. Carefully observe the administration site for signs of infection following injection and resolution of the opioid emergency.
There may be clinical settings, particularly the postpartum period in neonates with known or suspected exposure to maternal opioid use, where it is preferable to avoid the abrupt precipitation of opioid withdrawal symptoms. In these settings, consider use of an alternative, naloxone product which can be titrated to effect and, where applicable, dosed according to weight [see Use in Specific Populations (8.4)].
- 3 DOSAGE FORMS & STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Recurrent Respiratory and Central Nervous System Depression
The duration of action of most opioids may exceed that of EVZIO resulting in a return of respiratory and/or central nervous system depression after an initial improvement in symptoms. Therefore, it is necessary to seek emergency medical assistance immediately after delivering the first dose of EVZIO. Keep the patient under continued surveillance, and administer additional doses of EVZIO as necessary [see Dosage and Administration (2.2)]. Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance.
5.2 Risk of Limited Efficacy with Partial Agonists or Mixed Agonist/Antagonists
Reversal of respiratory depression by partial agonists or mixed agonist/antagonists such as buprenorphine and pentazocine, may be incomplete. Larger or repeat doses of naloxone hydrochloride may be required to antagonize buprenorphine because the latter has a long duration of action due to its slow rate of binding and subsequent slow dissociation from the opioid receptor [see Dosage and Administration (2.3)]. Buprenorphine antagonism is characterized by a gradual onset of the reversal effects and a decreased duration of action of the normally prolonged respiratory depression.
5.3 Precipitation of Severe Opioid Withdrawal
The use of EVZIO in patients who are opioid dependent may precipitate an acute abstinence syndrome characterized by the following signs and symptoms: body aches, diarrhea, tachycardia, fever, runny nose, sneezing, piloerection, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure. In neonates, opioid withdrawal may be life-threatening if not recognized and properly treated and may include the following signs and symptoms: convulsions, excessive crying and hyperactive reflexes. Monitor patients for the development of the signs and symptoms of opioid withdrawal.
Abrupt postoperative reversal of opioid depression after using naloxone hydrochloride may result in nausea, vomiting, sweating, tremulousness, tachycardia, hypotension, hypertension, seizures, ventricular tachycardia and fibrillation, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events. These events have primarily occurred in patients who had pre-existing cardiovascular disorders or received other drugs that may have similar adverse cardiovascular effects. Although a direct cause and effect relationship has not been established, after use of naloxone hydrochloride, monitor patients with pre-existing cardiac disease or patients who have received medications with potential adverse cardiovascular effects for hypotension, ventricular tachycardia or fibrillation, and pulmonary edema in an appropriate healthcare setting. It has been suggested that the pathogenesis of pulmonary edema associated with the use of naloxone hydrochloride is similar to neurogenic pulmonary edema, i.e., a centrally mediated massive catecholamine response leading to a dramatic shift of blood volume into the pulmonary vascular bed resulting in increased hydrostatic pressures.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
Precipitation of Severe Opioid Withdrawal [see Warnings and Precautions (5.3)]
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in practice.
The following adverse reactions were observed in EVZIO clinical studies. In two pharmacokinetic studies with a total of 54 healthy adult subjects exposed to 0.4 mg EVZIO, 0.8 mg EVZIO (two 0.4 mg EVZIOs) or 2 mg EVZIO, adverse reactions occurring in more than one subject were dizziness and injection site erythema.
The following adverse reactions have been identified during post-approval use of naloxone hydrochloride in the post-operative setting. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: Hypotension, hypertension, ventricular tachycardia and fibrillation, dyspnea, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events. Excessive doses of naloxone hydrochloride in post-operative patients have resulted in significant reversal of analgesia and have caused agitation [see Warnings and Precautions (5.3)].
Other events that have been reported in post-marketing use of EVZIO include agitation, disorientation, confusion, and anger.
Abrupt reversal of opioid effects in persons who were physically dependent on opioids has precipitated an acute withdrawal syndrome. Signs and symptoms have included: body aches, fever, sweating, runny nose, sneezing, piloerection, yawning, weakness, shivering or trembling, nervousness, restlessness or irritability, diarrhea, nausea or vomiting, abdominal cramps, increased blood pressure, tachycardia. In the neonate, opioid withdrawal signs and symptoms also included: convulsions, excessive crying, hyperactive reflexes [see Warnings and Precautions (5.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited available data on naloxone use in pregnant women are not sufficient to inform a drug-associated risk. However, there are risks to the fetus of the opioid-dependent mother with use of naloxone [see Clinical Considerations]. In animal reproduction studies, no embryotoxic or teratogenic effects were observed in mice and rats treated with naloxone hydrochloride during the period of organogenesis at doses equivalent to 4-times and 8-times, respectively, the dose of a 50 kg human given 10 mg [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal adverse reactions
Naloxone hydrochloride crosses the placenta, and may precipitate withdrawal in the fetus as well as in the opioid-dependent mother [see Warnings and Precautions (5.3)]. The fetus should be evaluated for signs of distress after EVZIO is used. Careful monitoring is needed until the fetus and mother are stabilized.
Data
Animal Data
Naloxone hydrochloride was administered during organogenesis to mice and rats at doses 4-times and 8-times, respectively, the dose of 10 mg/day given to a 50 kg human (when based on body surface area or mg/m2). These studies demonstrated no embryotoxic or teratogenic effects due to naloxone hydrochloride.
8.2 Lactation
Risk Summary
There is no information regarding the presence of naloxone in human milk, or the effects of naloxone on the breastfed infant or on milk production. Studies in nursing mothers have shown that naloxone does not affect prolactin or oxytocin hormone levels. Naloxone is minimally orally bioavailable. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for EVZIO and any potential adverse effects on the breastfed infant from EVZIO or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of EVZIO (for intramuscular and subcutaneous use) have been established in pediatric patients of all ages for the emergency treatment of known or suspected opioid overdose. Use of naloxone hydrochloride in all pediatric patients is supported by adult bioequivalence studies coupled with evidence from the safe and effective use of another naloxone hydrochloride injectable product. No pediatric studies were conducted for EVZIO.
Absorption of naloxone hydrochloride following subcutaneous or intramuscular administration in pediatric patients may be erratic or delayed. Even when the opiate-intoxicated pediatric patient responds appropriately to naloxone hydrochloride injection, he/she must be carefully monitored for at least 24 hours as a relapse may occur as naloxone is metabolized.
In opioid-dependent pediatric patients, (including neonates), administration of naloxone hydrochloride may result in an abrupt and complete reversal of opioid effects, precipitating an acute opioid withdrawal syndrome. There may be clinical settings, particularly the postpartum period in neonates with known or suspected exposure to maternal opioid use, where it is preferable to avoid the abrupt precipitation of opioid withdrawal symptoms. Unlike acute opioid withdrawal in adults, acute opioid withdrawal in neonates manifesting as seizures may be life-threatening if not recognized and properly treated. Other signs and symptoms in neonates may include excessive crying and hyperactive reflexes. In these settings where it may be preferable to avoid the abrupt precipitation of acute opioid withdrawal symptoms, consider use of an alternative, naloxone hydrochloride product that can be dosed according to weight and titrated to effect. [see Warnings and Precautions ( 5-5.3)].
In pediatric patients under the age of one year, the caregiver should pinch the thigh muscle while administering EVZIO. Carefully observe the administration site for evidence of residual needle parts, signs of infection, or both. [see Dosing Information ( 2-2.2)].
8.5 Geriatric Use
Geriatric patients have a greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy. Therefore, the systemic exposure of naloxone can be higher in these patients.
Clinical studies of naloxone hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
11 DESCRIPTION
EVZIO (naloxone hydrochloride injection, USP) is a pre-filled, single-use auto-injector. EVZIO is not made with natural rubber latex. Chemically, naloxone hydrochloride is the hydrochloride salt of 17-Allyl-4,5α-epoxy-3,14-dihydroxymorphinan-6-one hydrochloride with the following structure:
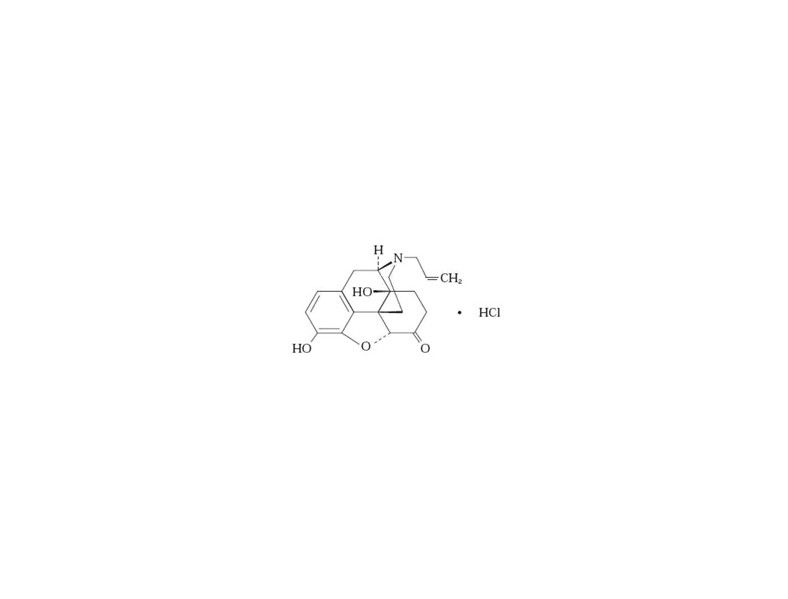
C19H21NO4• HCl
M.W. 363.84
Naloxone hydrochloride occurs as a white to slightly off-white powder, and is soluble in water, in dilute acids, and in strong alkali; slightly soluble in alcohol; practically insoluble in ether and in chloroform.
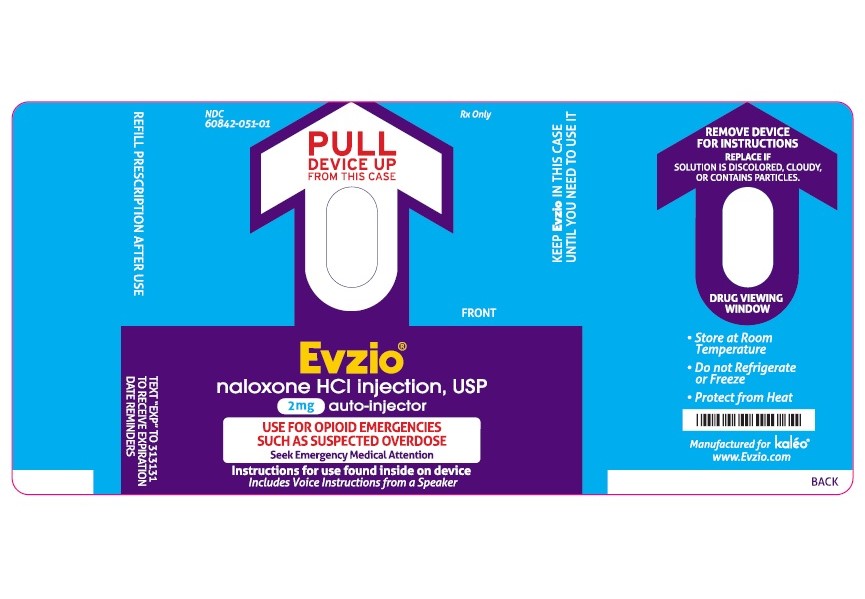
Each 0.4 mL of EVZIO contains 2 mg naloxone hydrochloride, 3.34 mg of sodium chloride, hydrochloric acid to adjust pH, and water for injection. The pH range is 3.0 to 4.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Naloxone hydrochloride is an opioid antagonist that antagonizes opioid effects by competing for the same receptor sites.
Naloxone hydrochloride reverses the effects of opioids, including respiratory depression, sedation, and hypotension. Also, it can reverse the psychotomimetic and dysphoric effects of agonist-antagonists such as pentazocine.
12.2 Pharmacodynamics
When naloxone hydrochloride is administered intravenously, the onset of action is generally apparent within two minutes. The time to onset of action is shorter for intravenous compared to subcutaneous or intramuscular routes of administration.
The duration of action is dependent upon the dose and route of administration of naloxone hydrochloride.
12.3 Pharmacokinetics
In a pharmacokinetic study in 30 healthy subjects, a single 0.4 mg subcutaneous or intramuscular naloxone injection administered using EVZIO provides equivalent naloxone AUC and 15% greater naloxone Cmax in comparison to a single 0.4 mg subcutaneous or intramuscular naloxone injection administered using a standard syringe.
Following a single 0.4 mg EVZIO injection, the median Tmax of naloxone was reached at 0.25 hours (range 0.08 to 1.23 hours), with a mean Cmax value of 1.24 (51.4% CV) ng/mL. The mean plasma half-life of naloxone in healthy adults was 1.28 (38.0% CV) hours. In the same study, following administration of a single dose of 0.4 mg naloxone injection using a standard syringe, the median Tmax was 0.33 hours (range 0.08 to 2.03 hours) and the mean Cmax value was 1.07 (45.1% CV) ng/mL. The mean plasma half-life was 1.36 (23.5% CV) hours.
A second pharmacokinetic study in 24 healthy subjects using a crossover design, evaluated a single 0.4 mg EVZIO injection, a single 2 mg EVZIO injection, and two 0.4 mg EVZIO injections administered two minutes apart (0.8 mg naloxone hydrochloride total). The pharmacokinetic parameters obtained in this study are shown in Table 1 and the plasma concentration time profiles of naloxone are in Figure 1.
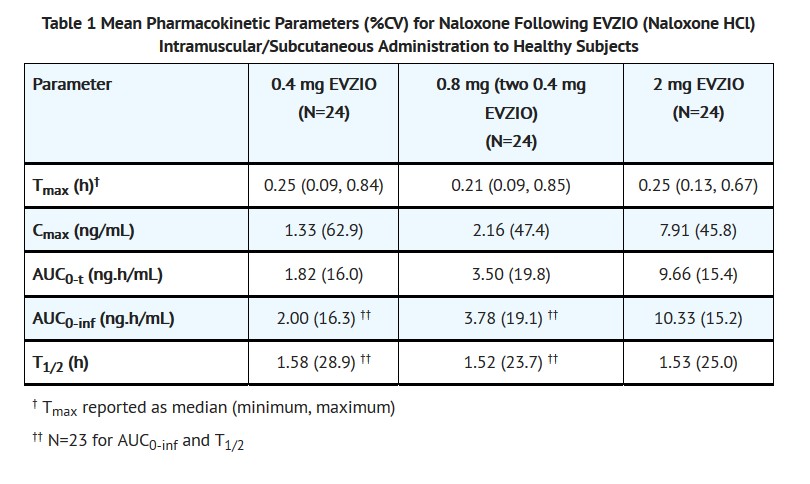
Figure 1 Mean ± SD Plasma Concentration of Naloxone, (a) 0-6 h and (b) 0-1h Following Intramuscular/Subcutaneous Administration using EVZIO
Distribution
Following parenteral administration, naloxone is distributed in the body and readily crosses the placenta. Plasma protein binding occurs but is relatively weak. Plasma albumin is the major binding constituent but significant binding of naloxone also occurs to plasma constituents other than albumin. It is not known whether naloxone is excreted into human milk.Elimination
Following a single 0.4 mg EVZIO injection, the mean plasma half-life of naloxone in healthy adults was 1.58 (28.9% CV) hours and 1.53 (25% CV) hours following a single 2 mg EVZIO injection. In a neonatal study of naloxone injection, the mean (± SD) plasma half-life was observed to be 3.1 (± 0.5) hours.Metabolism
Naloxone hydrochloride is metabolized in the liver, primarily by glucuronide conjugation with naloxone-3-glucoronide as the major metabolite.Excretion
After an oral or intravenous dose, about 25-40% of naloxone is excreted as metabolites in urine within 6 hours, about 50% in 24 hours, and 60-70% in 72 hours. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies to evaluate the carcinogenic potential of naloxone have not been completed.
Mutagenesis
Naloxone was weakly positive in the Ames mutagenicity and in the in vitro human lymphocyte chromosome aberration test but was negative in the in vitro Chinese hamster V79 cell HGPRT mutagenicity assay and in the in vivo rat bone marrow chromosome aberration study.
Impairment of Fertility
Reproduction studies conducted in mice and rats at doses 4-times and 8-times, respectively, the dose of a 50 kg human given 10 mg/day (when based on surface area or mg/m2), demonstrated no adverse effect of naloxone hydrochloride on fertility.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
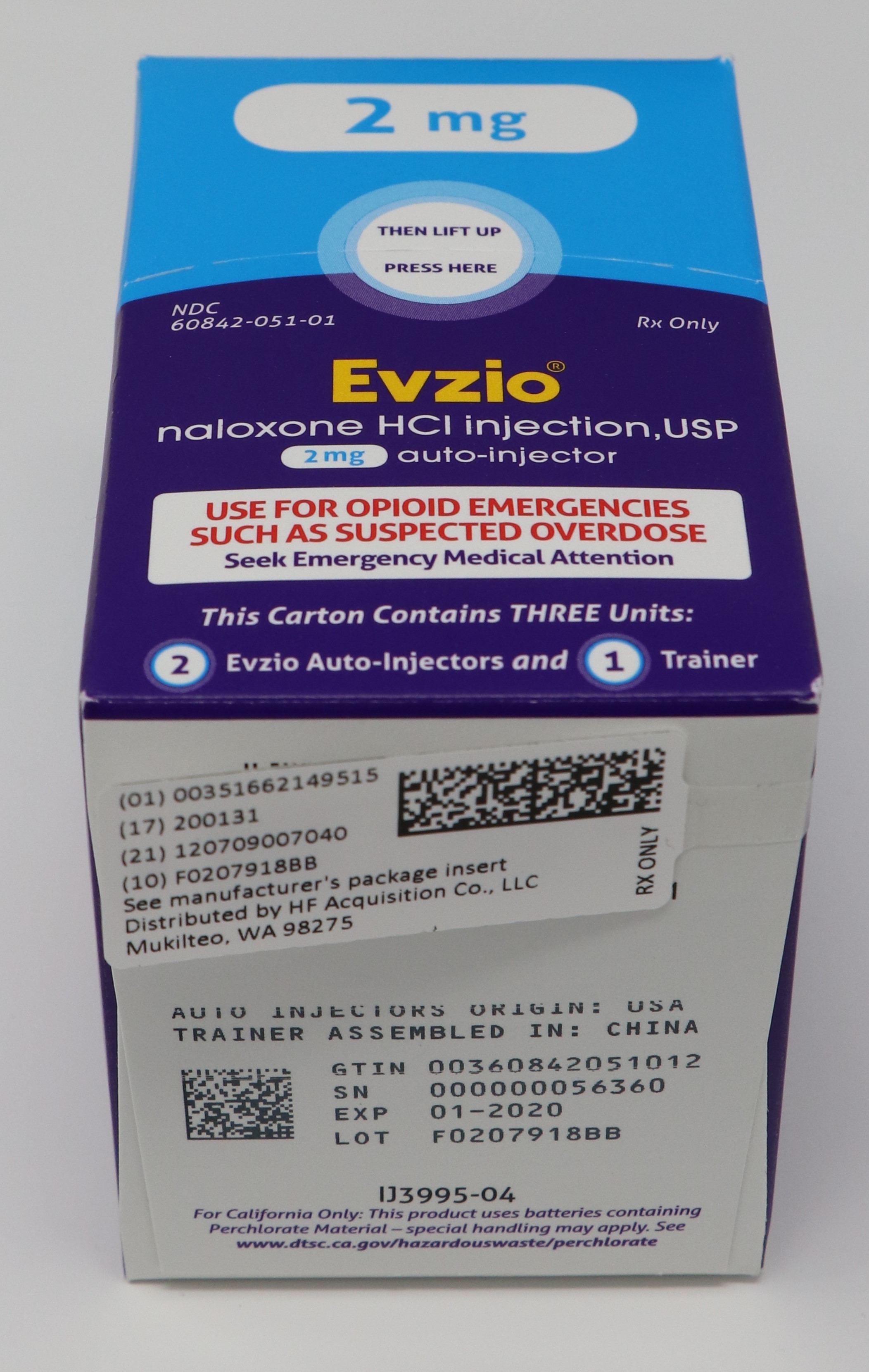
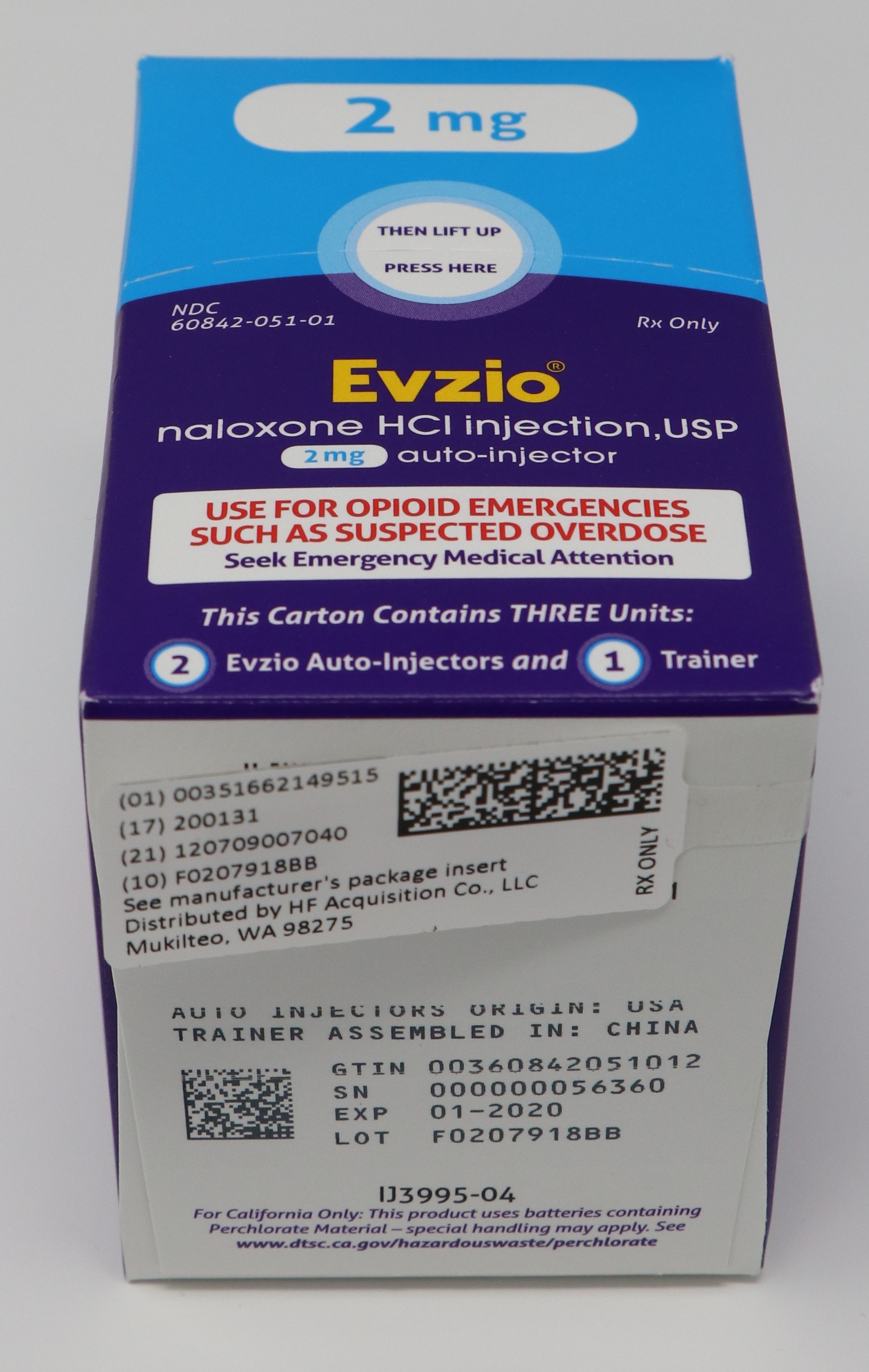
EVZIO(R) NALOXONE HCl INJECTION, USP is supplied in the following dosage forms.
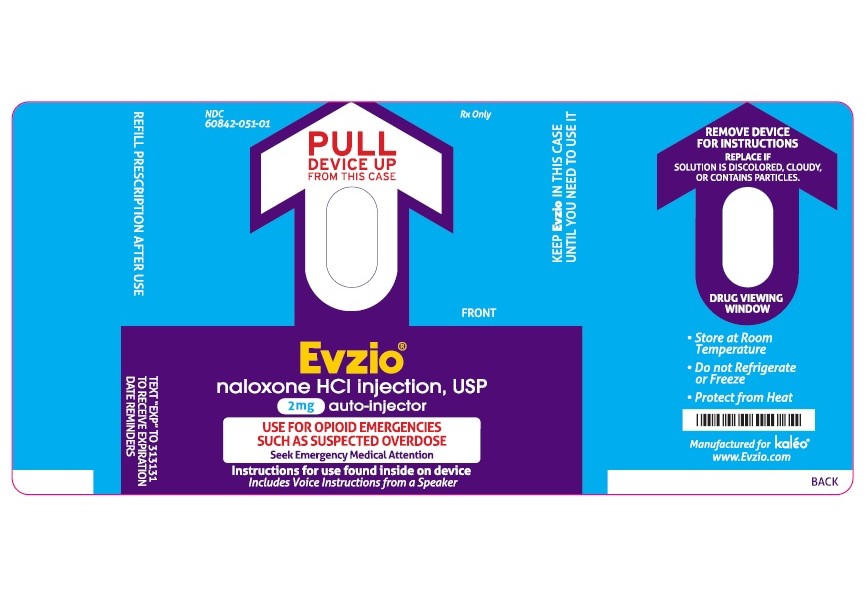
NDC 51662-1495-1
EVZIO(R) NALOXONE HCl INJECTION, USP 2mg AUTO-INJECTOR 2 PACKHF Acquisition Co LLC, DBA HealthFirst
Mukilteo, WA 98275Also supplied in the following manufacture supplied dosage forms
16.1 How Supplied
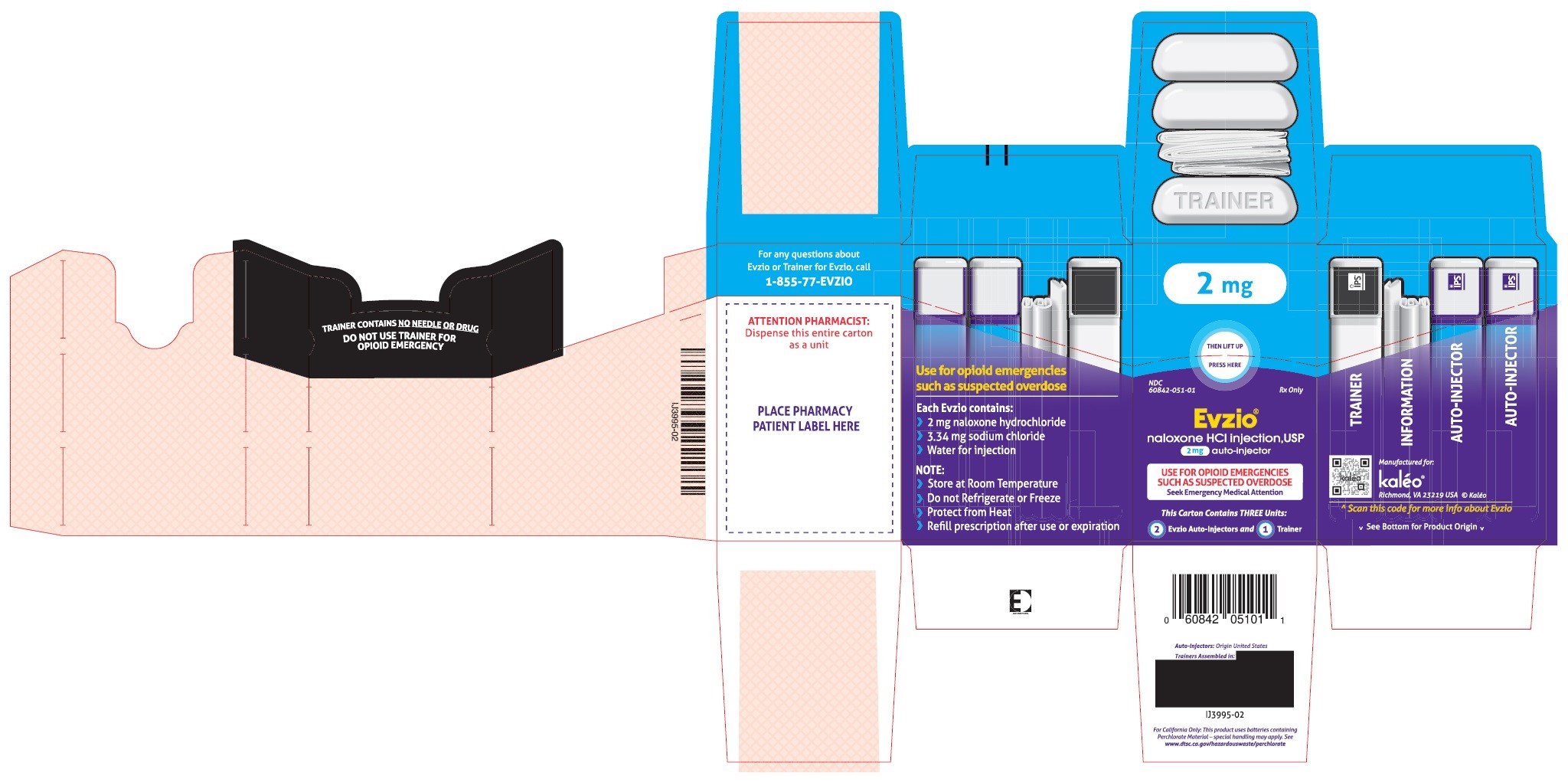
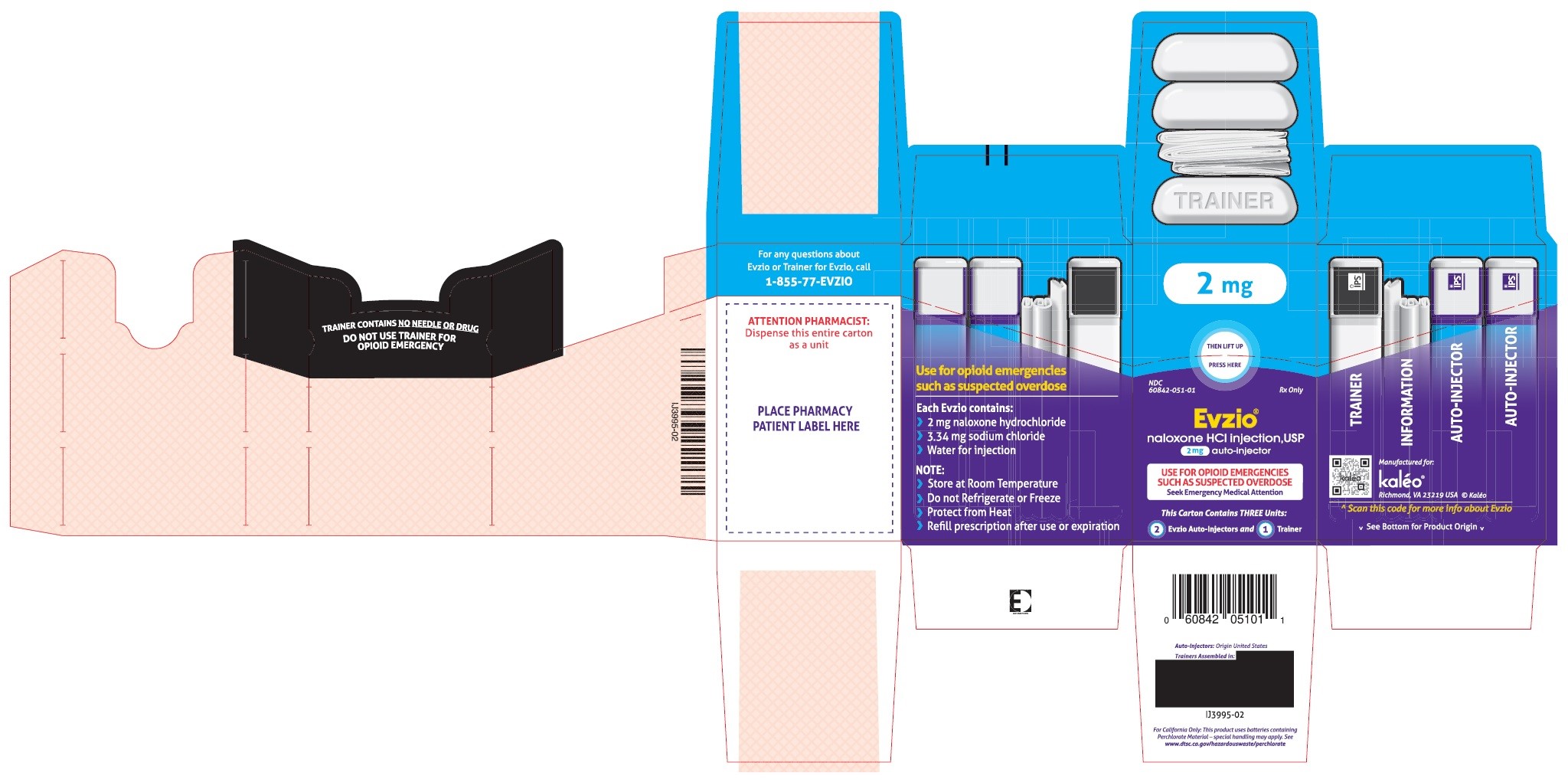
Carton containing two EVZIO (naloxone hydrochloride injection, USP) 2 mg auto-injectors and a single Trainer for EVZIO - NDC 60842-051-01
16.2 Storage and Handling
Store EVZIO in the outer case provided.
Store at controlled room temperature 15°C to 25°C (59°F to 77°F) excursions permitted between 4°C and 40°C (between 39°F and 104°F).
Before using, check to make sure the solution in the auto-injector is not discolored. Replace EVZIO if the solution is discolored or contains a precipitate.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and family members or caregivers to read the FDA-approved patient labeling ( Patient Information and Instructions for Use).
Instruct patients and their family members or caregivers to:
Become familiar with the following information contained in the carton as soon as they receive EVZIO:
EVZIO Instructions for Use
Trainer for EVZIO Instructions for Use
Trainer for EVZIOBecome familiar with the device labeling color scheme of EVZIO and the Trainer for EVZIO
The 2 mg dosage form of EVZIO is blue and purple.
The Trainer for EVZIO is black and white.Practice using the Trainer before EVZIO is needed.
Each EVZIO can only be used one time; however, Trainer for EVZIO can be re-used for training purposes and its red safety guard can be removed and replaced.
Both EVZIO and Trainer for EVZIO incorporate the electronic voice instruction system.It is recommended that patients and caregivers become familiar with the Trainer for EVZIO provided and read the Instructions for Use; however, untrained caregivers or family members should still attempt to use EVZIO during a suspected opioid overdose while awaiting definitive emergency medical care.
Recognition of Opioid Overdose
Instruct the patients and their family members or caregivers how to recognize the signs and symptoms of an opioid overdose requiring the use of EVZIO such as the following:
Extreme sleepiness - inability to awaken a patient verbally or upon a firm sternal rub.
Breathing problems - this can range from slow or shallow breathing to no breathing in a patient who cannot be awakened.
Other signs and symptoms that may accompany sleepiness and breathing problems include the following:Extremely small pupils (the black circle in the center of the colored part of the eye) sometimes called “pinpoint pupils.”
Slow heartbeat and/or low blood pressure.Risk of Recurrent Respiratory and Central Nervous System Depression
Instruct patients and their family members or caregivers that since the duration of action of most opioids may exceed that of EVZIO, they must seek immediate emergency medical assistance after the first dose of EVZIO and keep the patient under continued surveillance [see Dosage and Administration (2.2), Warnings and Precautions ( 5-5.3)].
Limited Efficacy for/with Partial Agonists or Mixed Agonist/Antagonists
Instruct patients and their family members or caregivers that the reversal of respiratory depression caused by partial agonists or mixed agonist/antagonists such as buprenorphine and pentazocine, may be incomplete and may require higher doses of naloxone hydrochloride or repeated administration of EVZIO [see Dosage and Administration ( 2-2.2), Warnings and Precautions ( 5-5.3)].
Precipitation of Severe Opioid Withdrawal
Instruct patients and their family members or caregivers that the use of EVZIO in patients who are opioid dependent may precipitate an acute abstinence syndrome characterized by the following signs and symptoms: body aches, diarrhea, tachycardia, fever, runny nose, sneezing, piloerection, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure. In neonates, opioid withdrawal may be life threatening if not recognized and properly treated, and may include the following signs and symptoms: convulsions, excessive crying, and hyperactive reflexes [see Warnings and Precautions ( 5-5.3, Adverse Reactions ( 6)].
Administration Instructions
Instruct patients and their family members or caregivers about the following important information:
Make sure EVZIO is present whenever persons may be intentionally or accidentally exposed to an opioid to treat serious opioid overdose (i.e., opioid emergencies).
Administer EVZIO as quickly as possible if a patient is unresponsive and an opioid overdose is suspected, even when in doubt, because prolonged respiratory depression may result in damage to the central nervous system or death. EVZIO is not a substitute for emergency medical care [see Dosage and Administration ( 2-2.1)].
EVZIO is user actuated and may be administered through clothing [e.g., pants, jeans, etc.] if necessary.
Inject EVZIO while pressing into the anterolateral aspect of the thigh. In pediatric patients less than 1 year of age, pinch the thigh muscle while administering EVZIO.
Upon actuation, EVZIO automatically inserts the needle intramuscularly or subcutaneously, delivers the naloxone, and retracts the needle fully into its housing. The needle is not visible before, during, or after injection.
Each EVZIO can only be used one time.
If the electronic voice instruction system on EVZIO does not work properly, EVZIO will still deliver the intended dose of naloxone hydrochloride when used according to the printed instructions on its label.
The electronic voice instructions are independent of activating EVZIO and it is not necessary to wait for the voice instructions to be completed prior to moving to the next step in the injection process.
Post-injection, the black base locks in place, a red indicator appears in the viewing window and electronic visual and audible instructions signal that EVZIO has delivered the intended dose of naloxone hydrochloride.
EVZIO’s red safety guard should not be replaced under any circumstances. However, the Trainer is designed for re-use and its red safety guard can be removed and replaced.
Periodically visually inspect the naloxone solution through the viewing window. If the solution is discolored, cloudy, or contains solid particles, replace it with a new EVZIO.
Replace EVZIO before its expiration date.---------------------------------------------------------------------------------------------------------------
Manufactured for:
kaleo, Inc.
Richmond, VA 23219*For California Only: This product uses batteries containing Perchlorate Material – special handling may apply. See www.dtsc.ca.gov/hazardouswaste/perchlorate
This product may be covered by one or more U.S. patents or pending patent applications. See www.kaleopharma.com/pat for details.
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
EVZIO® (EVV-zee-oh)
(naloxone hydrochloride injection)
Auto-Injector
for Intramuscular or Subcutaneous UseYou and your caregivers should read this Patient Information leaflet before an opioid emergency happens. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about EVZIO?
EVZIO is used to temporarily reverse the effects of opioid medicines. The medicine in EVZIO has no effect in people who are not taking opioid medicines. Always carry EVZIO with you in case of an opioid emergency.
Use EVZIO right away if you or your caregiver think signs or symptoms of an opioid emergency are present, even if you are not sure, because an opioid emergency can cause severe injury or death. Signs and symptoms of an opioid emergency may include:
unusual sleepiness and you are not able to awaken the person with a loud voice or rubbing firmly on the middle of their chest (sternum)
breathing problems including slow or shallow breathing in someone difficult to awaken or they look like they are not breathing
the black circle in the center of the colored part of the eye (pupil) is very small, sometimes called “pinpoint pupils” in someone difficult to awakenFamily members, caregivers, or other people who may have to use EVZIO in an opioid emergency should know where EVZIO is stored and how to give EVZIO before an opioid emergency happens.
Get emergency medical help right away after using the first dose of EVZIO. Rescue breathing or CPR (cardiopulmonary resuscitation) may be given while waiting for emergency medical help.
The signs and symptoms of an opioid emergency can return within several minutes after EVZIO is given. If this happens, give additional injections using a new EVZIO auto-injector every 2 to 3 minutes and continue to closely watch the person until emergency help is received.What is EVZIO?
EVZIO is a prescription medicine used in adults and children for the treatment of an opioid emergency such as an overdose or a possible opioid overdose with signs of breathing problems and severe sleepiness or not being able to respond.
EVZIO is to be given right away by a caregiver and does not take the place of emergency medical care. Get emergency medical help right away after the first dose of EVZIO, even if the person wakes up.
EVZIO is safe and effective in children for known or suspected opioid overdose.Who should not use EVZIO?
Do not use EVZIO if you are allergic to naloxone hydrochloride or any of the ingredients in EVZIO. See the end of this leaflet for a complete list of ingredients in EVZIO.
What should I tell my healthcare provider before using EVZIO?
Before using EVZIO, tell your healthcare provider about all of your medical conditions, including if you:
have heart problems
are pregnant or plan to become pregnant. Use of EVZIO may cause withdrawal symptoms in your unborn baby. Your unborn baby should be examined by a healthcare provider right away after you use EVZIO.Tell your healthcare provider about the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use EVZIO?
Read the “Instructions for Use” at the end of this Patient Information leaflet for detailed information about the right way to use EVZIO.
You should use EVZIO exactly as prescribed by your healthcare provider.
Each EVZIO auto-injector contains only 1 dose of medicine.
EVZIO should be injected into the muscle or skin of the outer thigh. It can be injected through clothing if needed.
Caregivers should pinch the thigh muscle while injecting EVZIO into a child under the age of one.
A Trainer for EVZIO with a separate “Trainer Instructions for Use” leaflet is included with EVZIO. For additional training information and video instructions go to www.EVZIO.com or call 1-855-773-8946.Practice with the Trainer for EVZIO before an opioid emergency happens to make sure you are able to safely use the real EVZIO in an emergency.
The Trainer for EVZIO does not contain a needle or medicine. It can be reused to practice your injection.
The red safety guard can be removed and replaced on the Trainer for EVZIO.What are the possible side effects of EVZIO?
EVZIO may cause serious side effects, including:
Sudden opioid withdrawal symptoms. In someone who has been using opioids regularly, opioid withdrawal symptoms can happen suddenly after receiving EVZIO and may include:
body aches
fever
sweating
runny nose
sneezing
goose bumps
yawning
weakness
shivering or trembling
nervousness
restlessness or irritability
diarrhea
nausea or vomiting
stomach cramping
increased blood pressure
increased heart rateCommon side effects of EVZIO include dizziness and injection site redness.
In infants under 4 weeks old who have been receiving opioids regularly, sudden opioid withdrawal may be life-threatening if not treated the right way. Signs and symptoms include: seizures, crying more than usual and increased reflexes. These are not all of the possible side effects of EVZIO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store EVZIO?
Store EVZIO at room temperature between 59°F to 77°F (15°C to 25°C).
Keep EVZIO in its outer case until ready to use.
Occasionally check EVZIO through the viewing window of the auto-injector. The solution should be clear. If the EVZIO solution is discolored, cloudy, or contains solid particles, replace it with a new EVZIO.
Your EVZIO has an expiration date. Replace it before the expiration date.Keep EVZIO and all medicines out of the reach of children.
General information about the safe and effective use of EVZIO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use EVZIO for a condition for which it was not prescribed. You can ask your pharmacist or healthcare provider for information about EVZIO that is written for health professionals.
What are the ingredients in EVZIO?
Active ingredient: naloxone hydrochloride
Inactive ingredients: sodium chloride, hydrochloric acid to adjust pH, and water
EVZIO is not made with natural rubber latex.
Manufactured for kaleo, Inc., Richmond, VA, 23219
For more information, go to www.EVZIO.com or call 1-855-773-8946This Patient Information has been approved by the U.S. Food and Drug Administration.Revised: 10/2016
-
INSTRUCTIONS FOR USE
Instructions for Use
EVZIO® (EVV-zee-oh)
(naloxone hydrochloride injection)
Auto-InjectorRead the Instructions for Use that comes with EVZIO before using it. Talk to your healthcare provider if you or your caregivers have any questions about the use of EVZIO.
Automated voice instructions
EVZIO has a speaker that provides voice instructions to help guide you through each step of the injection. See Figure A. If the voice instructions do not work for any reason, EVZIO will still work. If this happens, use EVZIO as instructed below and follow the written instructions on the EVZIO auto-injector label.
How to use EVZIO

How to know that EVZIO has been used. See Figure E.
The Black base will lock into place.
The voice instruction system will state that EVZIO has been used and the LED will blink red.
The Red safety guard cannot be replaced.
The viewing window will no longer be clear. You will see a red indicator.
What to do after EVZIO has been used:
Get emergency medical help right away.
Put the used EVZIO back into its outer case.
Do not throw away the EVZIO in household trash. Do not recycle EVZIO.
Used EVZIO should be taken to a healthcare setting for proper disposal in a sharps container.There may be local or state laws about how to throw away used auto-injectors.*
*For California Only: This product uses batteries containing Perchlorate Material – special handling may apply. See www.dtsc.ca.gov/hazardouswaste/perchlorate
How should I store EVZIO?
Store EVZIO at room temperature between 59°F to 77°F (15°C to 25°C).
Keep EVZIO in its outer case until ready to use.
Occasionally check EVZIO through the viewing window of the auto-injector. The solution should be clear. If the EVZIO solution is discolored, cloudy, or contains solid particles, replace it with a new EVZIO.
Your EVZIO has an expiration date. Replace it before the expiration date.Keep EVZIO and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for: kaleo, Inc., Richmond, VA 23219
Revised: 10/2016
-
INSTRUCTIONS FOR USE -TRAINER
Trainer for EVZIO®
Trainer Instructions for UseImportant:
The Trainer for EVZIO Does Not contain a needle or medicine. Always carry your real EVZIO with you in case of an opioid emergency.
Tell your family, friends, co-workers or other individuals who may need to use EVZIO during an opioid emergency, where you keep your EVZIO.
Important Information about the Trainer for EVZIO:
Inside your Trainer for EVZIO are:
batteries
a speaker that will make a beeping sound and that produces electronic voice instructions
red and green blinking lightsThe Trainer for EVZIO batteries are made to last for over 1,000 demonstrations or practices.
If the electronic voice instructions do not work properly, the Trainer for EVZIO can still be used for demonstration or practice. If this happens, use the instructions below and follow the written instructions on the Trainer for EVZIO label.
What is the Trainer for EVZIO?
The Trainer for EVZIO does not contain a needle or medicine and can be reused to practice your injection.
Practice with the Trainer for EVZIO before an opioid emergency happens to make sure you are able to safely use the real EVZIO in an emergency.
A Trainer for EVZIO comes with each EVZIO prescription so that you and your caregiver can practice and demonstrate how to use EVZIO.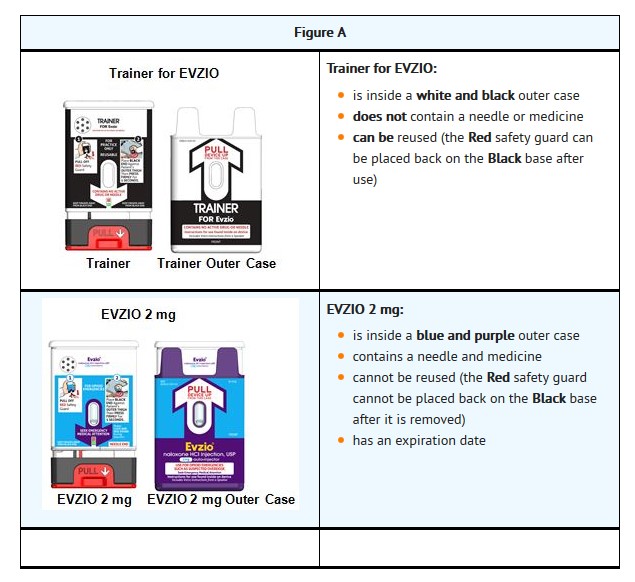
In case of an opioid overdose or possible opioid overdose emergency, use the real EVZIO and not the Trainer for EVZIO.
Who should practice using the Trainer for EVZIO?
Anyone who may need to help you with EVZIO in case of an opioid overdose or possible overdose emergency should practice using the Trainer for EVZIO.
Have them practice using the Trainer for EVZIO and review the Patient Information leaflet included in the packaging with your prescription of EVZIO.
For more information and video instructions on the use of EVZIO, go to http://www.EVZIO.com or call 1-855-77-EVZIO.
Practicing with the Trainer for EVZIO
Practice with the Trainer for EVZIO before an opioid emergency happens to make sure you are able to safely use the real EVZIO in the case of an opioid overdose or possible overdose emergency.
You and your caregivers should practice every day for the first week after you receive your Trainer for EVZIO, and then at least 1 time each week, to help you feel familiar with using EVZIO quickly and safely during an opioid overdose or possible opioid overdose emergency. Even when you are familiar with using the Trainer for EVZIO, continue to practice using it often.How to use the Trainer for EVZIO
Even though the Trainer for EVZIO does not have a needle and contains no medicine, it works the same way as the real EVZIO.
Just like the real EVZIO, the Trainer for EVZIO contains an electronic voice instruction system to help guide you through each step of the injection. If the voice instructions do not work for any reason, you can still use the Trainer for EVZIO to practice using the instructions below and following the written instructions on the Trainer for EVZIO.
The Trainer for EVZIO has the same blinking red and green lights as the real EVZIO. These blinking lights help provide visual cues for each voice instruction and step.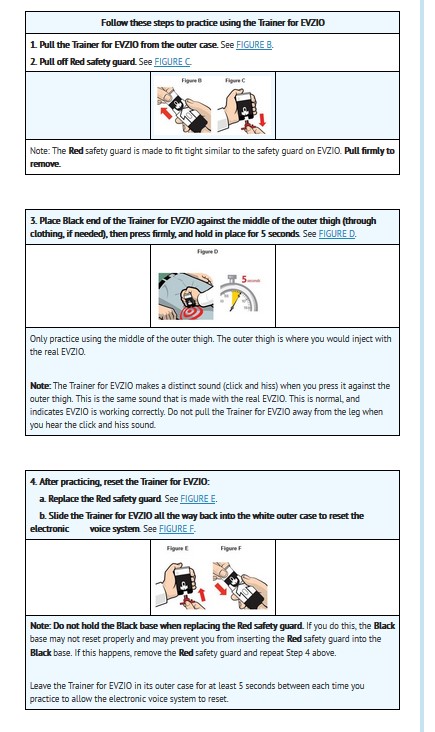
How should I dispose of the Trainer for EVZIO?
The Trainer for EVZIO contains electronics and lithium coin cell batteries, and should be disposed of in the correct manner. Follow your State and local environmental regulations for disposal.
For California Only: This product uses batteries containing Perchlorate Material- special handling may apply. See www.dtsc.ca.gov/hazardouswaste/perchlorate
For more information or questions about the Trainer for EVZIO, go to www.EVZIO.com or call 1-855-773-8946.
How should I store the Trainer for EVZIO?
Store the Trainer for EVZIO at room temperature between 59°F to 77°F (15°C to 25°C).
Store the Trainer for EVZIO in its outer case.This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for kaleo, Inc. Richmond, VA 23219
Revised: 10/2016
- PRINCIPAL DISPLAY PANEL - SERIALIZED CARTON LABELING
- PRINCIPAL DISPLAY PANEL -CARTON LABEL
- PRINCIPAL DISPLAY PANEL - AUTO INJECTOR CASE LABEL
- PRINCIPAL DISPLAY PANEL - AUTO INJECTOR LABEL
-
INGREDIENTS AND APPEARANCE
EVZIO(R) NALOXONE HCL
evzio(r) naloxone hcl injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51662-1495(NDC:60842-051) Route of Administration INTRAMUSCULAR, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE HYDROCHLORIDE 2 mg in 0.4 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) 3.34 mg in 0.4 mL HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51662-1495-1 2 in 1 CARTON 01/11/2020 1 0.4 mL in 1 DOSE PACK; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209862 01/11/2020 Labeler - HF Acquisition Co LLC, DBA HealthFirst (045657305) Registrant - HF Acquisition Co LLC, DBA HealthFirst (045657305) Establishment Name Address ID/FEI Business Operations HF Acquisition Co LLC, DBA HealthFirst 045657305 relabel(51662-1495)