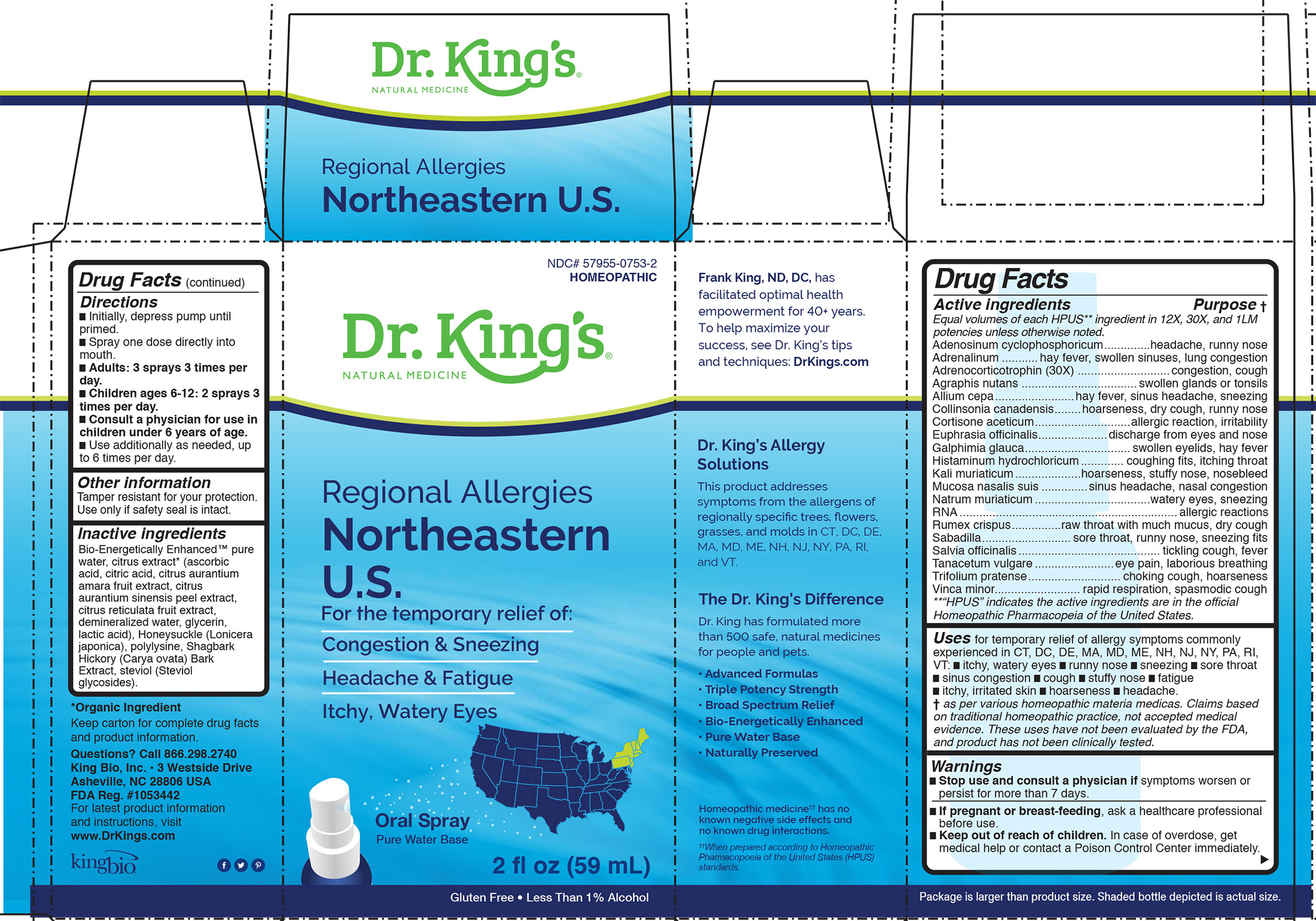
Label: REGIONAL ALLERGIES NORTHEASTERN US- adenosinum cyclophosphoricum, adrenalinum, adrenocorticotrophin, agraphis nutans, allium cepa, collinsonia canadensis, cortisone aceticum, euphrasia officinalis, galphimia glauca, histaminum hydrochloricum, kali muriaticum, mucosa nasalis suis, natrum muriaticum, rna, rumex crispus, sabadilla, salvia officinalis, tanacetum vulgare, trifolium pratense, vinca minor liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 57955-0753-2 - Packager: King Bio Inc,
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 8, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
HPUS active ingredients
Equal volumes of each ingredient in 12X, 30X, and 1LM potencies.
Adenosinum cyclophosphoricum, Adrenalinum, Adrenocorticotrophin, Agraphis nutans, Allium cepa, Collinsonia canadensis, Cortisone aceticum, Euphrasia officinalis, Galphimia glauca, Histaminum hydrochloricum, Kali muriaticum, Mucosa nasalis suis, Natrum muriaticum, RNA, Rumex crispus, Sabadilla, Salvia officinalis, Tanacetum vulgare, Trifolium pratense, Vinca minor.
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients
Bio-Energetically Enhanced™ pure
water, citrus extract* (ascorbic
acid, citric acid, citrus aurantium
amara fruit extract, citrus
aurantium sinensis peel extract,
citrus reticulata fruit extract,
demineralized water, glycerin,
lactic acid), Honeysuckle (Lonicera
japonica), polylysine, Shagbark
Hickory (Carya ovata) Bark
Extract, steviol (Steviol
glycosides). -
PURPOSE
Drug Facts__________________________________________________________________________________________________________
HPUS active ingredients Purpose
Equal volumes ofeach ingredient in 12X, 30X, and LM1 potencies.
Adenosinum cyclophosphoricum.............................................headache, runny nose
Adrenalinum........................................................................hay fever, swollen sinuses, lung congestion
Adrenocorticotrophin (30X)....................................................congestion, cough
Agraphis nutans....................................................................swollen glands or tonsils
Allium cepa...........................................................................hay fever, sinus headache, sneezing
Collinsonia canadensis............................................................hoarseness, dry cough, runny nose
Cortisone aceticum................................................................allergic reaction, irritability
Euphrasia officinalis...............................................................discharge from eyes and nose
Galphimia glauca...................................................................swollen eyelids, hay fever
Histaminum hydrochloricum....................................................coughing fits, itching throat
Kali muriaticum.....................................................................hoarseness, stuffy nose, nosebleed
Mucosa nasalis suis................................................................sinus headache, nasal congestion
Natrum muriaticum................................................................watery eyes, sneezing
RNA.....................................................................................allergic reactions
Rumex crispus.......................................................................raw throat with much mucus, dry cough
Sabadilla...............................................................................sore throat, runny nose, sneezing fits
Salvia officinalis.....................................................................tickling cough, fever
Tanacetum vulgare.................................................................eye pain, laborious breathing
Trifolium pratense..................................................................choking cough, hoarseness
Vinca minor...........................................................................rapid respiration, spasmodic cough
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
REGIONAL ALLERGIES NORTHEASTERN US
adenosinum cyclophosphoricum, adrenalinum, adrenocorticotrophin, agraphis nutans, allium cepa, collinsonia canadensis, cortisone aceticum, euphrasia officinalis, galphimia glauca, histaminum hydrochloricum, kali muriaticum, mucosa nasalis suis, natrum muriaticum, rna, rumex crispus, sabadilla, salvia officinalis, tanacetum vulgare, trifolium pratense, vinca minor liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-0753 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADENOSINE CYCLIC PHOSPHATE (UNII: E0399OZS9N) (ADENOSINE CYCLIC PHOSPHATE - UNII:E0399OZS9N) ADENOSINE CYCLIC PHOSPHATE 12 [hp_X] in 59 mL EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 12 [hp_X] in 59 mL CORTICOTROPIN (UNII: K0U68Q2TXA) (CORTICOTROPIN - UNII:K0U68Q2TXA) CORTICOTROPIN 12 [hp_X] in 59 mL HYACINTHOIDES NON-SCRIPTA (UNII: 5F658PFU56) (HYACINTHOIDES NON-SCRIPTA - UNII:5F658PFU56) HYACINTHOIDES NON-SCRIPTA 12 [hp_X] in 59 mL ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 12 [hp_X] in 59 mL COLLINSONIA CANADENSIS ROOT (UNII: O2630F3XDR) (COLLINSONIA CANADENSIS ROOT - UNII:O2630F3XDR) COLLINSONIA CANADENSIS ROOT 12 [hp_X] in 59 mL CORTISONE ACETATE (UNII: 883WKN7W8X) (CORTISONE - UNII:V27W9254FZ) CORTISONE ACETATE 12 [hp_X] in 59 mL EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 12 [hp_X] in 59 mL GALPHIMIA GLAUCA FLOWERING TOP (UNII: 93PH5Q8M7E) (GALPHIMIA GLAUCA FLOWERING TOP - UNII:93PH5Q8M7E) GALPHIMIA GLAUCA FLOWERING TOP 12 [hp_X] in 59 mL HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 12 [hp_X] in 59 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 12 [hp_X] in 59 mL SUS SCROFA NASAL MUCOSA (UNII: ID3Z1X61WY) (SUS SCROFA NASAL MUCOSA - UNII:ID3Z1X61WY) SUS SCROFA NASAL MUCOSA 12 [hp_X] in 59 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 12 [hp_X] in 59 mL SACCHAROMYCES CEREVISIAE RNA (UNII: J17GBZ5VGX) (SACCHAROMYCES CEREVISIAE RNA - UNII:J17GBZ5VGX) SACCHAROMYCES CEREVISIAE RNA 12 [hp_X] in 59 mL RUMEX CRISPUS ROOT (UNII: 9N1RM2S62C) (RUMEX CRISPUS ROOT - UNII:9N1RM2S62C) RUMEX CRISPUS ROOT 12 [hp_X] in 59 mL SCHOENOCAULON OFFICINALE SEED (UNII: 6NAF1689IO) (SCHOENOCAULON OFFICINALE SEED - UNII:6NAF1689IO) SCHOENOCAULON OFFICINALE SEED 12 [hp_X] in 59 mL SAGE (UNII: 065C5D077J) (SAGE - UNII:065C5D077J) SAGE 12 [hp_X] in 59 mL TANACETUM VULGARE TOP (UNII: D52957JQ8M) (TANACETUM VULGARE TOP - UNII:D52957JQ8M) TANACETUM VULGARE TOP 12 [hp_X] in 59 mL TRIFOLIUM PRATENSE FLOWER (UNII: 4JS0838828) (TRIFOLIUM PRATENSE FLOWER - UNII:4JS0838828) TRIFOLIUM PRATENSE FLOWER 12 [hp_X] in 59 mL VINCA MINOR (UNII: WGM46PQF02) (VINCA MINOR - UNII:WGM46PQF02) VINCA MINOR 12 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARYA OVATA BARK (UNII: X765CF609L) CITRUS BIOFLAVONOIDS (UNII: BD70459I50) LONICERA JAPONICA FLOWER (UNII: 4465L2WS4Y) POLYEPSILON-LYSINE (4000 MW) (UNII: WB0M8X4TWR) REBAUDIOSIDE A (UNII: B3FUD0528F) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-0753-2 1 in 1 CARTON 02/21/2018 1 59 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/21/2018 Labeler - King Bio Inc, (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 api manufacture(57955-0753) , manufacture(57955-0753)