Label: YESCARTA- axicabtagene ciloleucel suspension
- NDC Code(s): 71287-119-01, 71287-119-02
- Packager: Kite Pharma, Inc.
- Category: CELLULAR THERAPY
Drug Label Information
Updated June 19, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use YESCARTA safely and effectively. See full prescribing information for YESCARTA.
YESCARTA® (axicabtagene ciloleucel) suspension for intravenous infusion
Initial U.S. Approval: 2017WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES and SECONDARY HEMATOLOGICAL MALIGNANCIES
See full prescribing information for complete boxed warning.
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients receiving YESCARTA. Do not administer YESCARTA to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids (2.2, 2.3, 5.1).
- Neurologic toxicities, including fatal or life-threatening reactions, occurred in patients receiving YESCARTA, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with YESCARTA. Provide supportive care and/or corticosteroids, as needed (2.2, 2.3, 5.2).
- T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies, including YESCARTA (5.8).
- YESCARTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA and TECARTUS REMS (5.3).
RECENT MAJOR CHANGES
Boxed Warning 04/2024 Dosage and Administration (2.2) 06/2024 Warnings and Precautions (5.3) 06/2024 Warnings and Precautions, Secondary Malignancies (5.8) 04/2024 INDICATIONS AND USAGE
YESCARTA is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of:
- Adult patients with large B-cell lymphoma that is refractory to first-line chemoimmunotherapy or that relapses within 12 months of first-line chemoimmunotherapy. (1.1)
- Adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma. (1.1)
Limitations of Use: YESCARTA is not indicated for the treatment of patients with primary central nervous system lymphoma. (1.1)
- Adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy. This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s). (1.2)
DOSAGE AND ADMINISTRATION
For autologous use only. For intravenous use only.
- Do NOT use a leukodepleting filter. (2.2)
- Administer a lymphodepleting regimen of cyclophosphamide and fludarabine before infusion of YESCARTA. (2.2)
- Verify the patient's identity prior to infusion. (2.2)
- Premedicate with acetaminophen and an H1-antihistamine. (2.2)
- Confirm availability of tocilizumab prior to infusion. (2.1, 5.1)
- Dosing of YESCARTA is based on the number of chimeric antigen receptor (CAR)-positive viable T cells. (2.1)
- The target YESCARTA dose is 2 × 106 CAR-positive viable T cells per kg body weight, with a maximum of 2 × 108 CAR-positive viable T cells. (2.1)
- Administer YESCARTA in a certified healthcare facility. (2.2, 5.1, 5.2, 5.3)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- None. (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: Monitor for hypersensitivity reactions during infusion. (5.4)
- Serious Infections: Monitor patients for signs and symptoms of infection; treat appropriately. (5.5)
- Prolonged Cytopenias: Patients may exhibit Grade 3 or higher cytopenias for several weeks following YESCARTA infusion. Monitor complete blood counts. (5.6)
- Hypogammaglobulinemia: Monitor and provide replacement therapy. (5.7)
- Secondary Malignancies: T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies, including YESCARTA (5.8). In the event that a secondary malignancy occurs after treatment with YESCARTA, contact Kite at 1-844-454-KITE (5483). (5.8)
- Effects on Ability to Drive and Use Machines: Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, for at least 8 weeks after receiving YESCARTA. (5.9)
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥ 30%), excluding laboratory abnormalities, in patients with non-Hodgkin lymphoma are CRS, fever, hypotension, encephalopathy, fatigue, tachycardia, headache, nausea, febrile neutropenia, diarrhea, musculoskeletal pain, infections with pathogen unspecified, chills and decreased appetite. (6.1)
The most common Grade 3-4 laboratory abnormalities (≥ 30%) are leukopenia, lymphopenia, neutropenia, anemia, thrombocytopenia, and hypophosphatemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Kite at 1-844-454-KITE (5483) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES and SECONDARY HEMATOLOGICAL MALIGNANCIES
1 INDICATIONS AND USAGE
1.1 Large B-cell Lymphoma
1.2 Follicular Lymphoma
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Administration
2.3 Management of Severe Adverse Reactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome
5.2 Neurologic Toxicities
5.3 YESCARTA and TECARTUS REMS
5.4 Hypersensitivity Reactions
5.5 Serious Infections
5.6 Prolonged Cytopenias
5.7 Hypogammaglobulinemia
5.8 Secondary Malignancies
5.9 Effects on Ability to Drive and Use Machines
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Relapsed or Refractory Large B-Cell Lymphoma
14.2 Relapsed or Refractory Follicular Lymphoma
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES and SECONDARY HEMATOLOGICAL MALIGNANCIES
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients receiving YESCARTA. Do not administer YESCARTA to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.1)].
- Neurologic toxicities, including fatal or life-threatening reactions, occurred in patients receiving YESCARTA, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with YESCARTA. Provide supportive care and/or corticosteroids as needed [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.2)].
- T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies, including YESCARTA [see Warnings and Precautions (5.8)].
- YESCARTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA and TECARTUS REMS [see Warnings and Precautions (5.3)].
-
1 INDICATIONS AND USAGE
1.1 Large B-cell Lymphoma
YESCARTA is indicated for the treatment of:
- Adult patients with large B-cell lymphoma that is refractory to first-line chemoimmunotherapy or that relapses within 12 months of first-line chemoimmunotherapy.
- Adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.
1.2 Follicular Lymphoma
YESCARTA is indicated for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy.
This indication is approved under accelerated approval based on response rate [see Clinical Studies (14.2)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).
-
2 DOSAGE AND ADMINISTRATION
For autologous use only. For intravenous use only.
2.1 Dose
Each single infusion bag of YESCARTA contains a suspension of chimeric antigen receptor (CAR)-positive T cells in approximately 68 mL. The target dose is 2 × 106 CAR-positive viable T cells per kg body weight, with a maximum of 2 × 108 CAR-positive viable T cells.
2.2 Administration
YESCARTA is for autologous use only. The patient's identity must match the patient identifiers on the YESCARTA cassette and infusion bag. Do not infuse YESCARTA if the information on the patient-specific label does not match the intended patient.
Preparing Patient for YESCARTA Infusion
Confirm availability of YESCARTA prior to starting the lymphodepleting regimen.
Pre-treatment
- Administer a lymphodepleting chemotherapy regimen of cyclophosphamide 500 mg/m2 intravenously and fludarabine 30 mg/m2 intravenously on the fifth, fourth, and third day before infusion of YESCARTA.
Premedication
- Administer acetaminophen 650 mg PO and diphenhydramine 12.5 mg intravenously or PO approximately 1 hour before YESCARTA infusion.
- Consider the use of prophylactic corticosteroid in patients after weighing the potential benefits and risks [see Warnings and Precautions (5.1 and 5.2)].
Preparation of YESCARTA for Infusion
Coordinate the timing of YESCARTA thaw and infusion. Confirm the infusion time in advance, and adjust the start time of YESCARTA thaw such that it will be available for infusion when the patient is ready.
- Confirm patient identity: Prior to YESCARTA preparation, match the patient's identity with the patient identifiers on the YESCARTA cassette.
- Do not remove the YESCARTA product bag from the cassette if the information on the patient-specific label does not match the intended patient.
- Once patient identification is confirmed, remove the YESCARTA product bag from the cassette and check that the patient information on the cassette label matches the bag label.
- Inspect the product bag for any breaches of container integrity such as breaks or cracks before thawing. If the bag is compromised, follow the local guidelines (or call Kite at 1-844-454-KITE).
- Place the infusion bag inside a second sterile bag per local guidelines.
- Thaw YESCARTA at approximately 37°C using either a water bath or dry thaw method until there is no visible ice in the infusion bag. Gently mix the contents of the bag to disperse clumps of cellular material. If visible cell clumps remain continue to gently mix the contents of the bag. Small clumps of cellular material should disperse with gentle manual mixing. Do not wash, spin down, and/or re-suspend YESCARTA in new medium prior to infusion.
- Once thawed, YESCARTA may be stored at room temperature (20°C to 25°C) for up to 3 hours.
Administration
- For autologous use only.
- Ensure that tocilizumab and emergency equipment are available prior to infusion and during the recovery period.
- Do NOT use a leukodepleting filter.
- Central venous access is recommended for the infusion of YESCARTA.
- Confirm the patient's identity matches the patient identifiers on the YESCARTA product bag.
- Prime the tubing with normal saline prior to infusion.
- Infuse the entire contents of the YESCARTA bag within 30 minutes by either gravity or a peristaltic pump. YESCARTA is stable at room temperature for up to 3 hours after thaw.
- Gently agitate the product bag during YESCARTA infusion to prevent cell clumping.
- After the entire content of the product bag is infused, rinse the tubing with normal saline at the same infusion rate to ensure all product is delivered.
YESCARTA contains human blood cells that are genetically modified with replication incompetent retroviral vector. Follow universal precautions and local biosafety guidelines for handling and disposal to avoid potential transmission of infectious diseases.
Monitoring
- Administer YESCARTA at a certified healthcare facility.
- Monitor patients at least daily for 7 days at the certified healthcare facility following infusion for signs and symptoms of CRS and neurologic toxicities.
- Instruct patients to remain within proximity of a certified healthcare facility for at least 4 weeks following infusion.
2.3 Management of Severe Adverse Reactions
Cytokine Release Syndrome (CRS)
Identify CRS based on clinical presentation [see Warnings and Precautions (5.1)]. Evaluate for and treat other causes of fever, hypoxia, and hypotension. If CRS is suspected, manage according to the recommendations in Table 1. Patients who experience Grade 2 or higher CRS (e.g., hypotension not responsive to fluids, or hypoxia requiring supplemental oxygenation) should be monitored with continuous cardiac telemetry and pulse oximetry. For patients experiencing severe CRS, consider performing an echocardiogram to assess cardiac function. For severe or life-threatening CRS, consider intensive-care supportive therapy.
Table 1. CRS Grading and Management Guidance CRS Grade * Tocilizumab Corticosteroids Grade 1
Symptoms require symptomatic treatment only (e.g., fever, nausea, fatigue, headache, myalgia, malaise).If symptoms (e.g., fever) not improving after 24 hours, consider managing as Grade 2. If not improving after 3 days, administer one dose of dexamethasone 10 mg intravenously. Grade 2
Symptoms require and respond to moderate intervention.
Oxygen requirement less than 40% FiO2 or hypotension responsive to fluids or low-dose of one vasopressor or
Grade 2 organ toxicity.†Administer tocilizumab‡ 8 mg/kg intravenously over 1 hour (not to exceed 800 mg).
If no clinical improvement in the signs and symptoms of CRS after the first dose, repeat tocilizumab every 8 hours as needed.
Limit to a maximum of 3 doses in a 24-hour period; maximum total of 4 doses.
If improving, discontinue tocilizumab.Administer dexamethasone 10 mg intravenously once daily.
If improving, manage as Grade 1 above and continue corticosteroids until the severity is Grade 1 or less, then quickly taper as clinically appropriate.
If not improving, manage as appropriate grade below.Grade 3
Symptoms require and respond to aggressive intervention.
Oxygen requirement greater than or equal to 40% FiO2 or hypotension requiring high-dose or multiple vasopressors or
Grade 3 organ toxicity or Grade 4 transaminitis.Per Grade 2.
If improving, manage as appropriate grade above.Dexamethasone 10 mg intravenously three times a day.
If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then quickly taper as clinically appropriate.
If not improving, manage as Grade 4.Grade 4
Life-threatening symptoms.
Requirements for ventilator support, continuous veno-venous hemodialysis (CVVHD) or
Grade 4 organ toxicity (excluding transaminitis).Per Grade 2.
If improving, manage as appropriate grade above.Administer methylprednisolone 1000 mg intravenously once per day for 3 days.
If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then taper as clinically appropriate.
If not improving, consider methylprednisolone 1000 mg 2-3 times a day or alternate therapy.§Neurologic Toxicity
Monitor patients for signs and symptoms of neurologic toxicity/immune effector cell-associated neurotoxicity syndrome (ICANS) (Table 2). Rule out other causes of neurologic symptoms. Patients who experience Grade 2 or higher neurologic toxicities/ICANS should be monitored with continuous cardiac telemetry and pulse oximetry. Provide intensive-care supportive therapy for severe or life-threatening neurologic toxicities. Consider levetiracetam for seizure prophylaxis for any grade of neurologic toxicities.
Table 2. Neurologic Toxicity/ICANS Grading and Management Guidance Grading Assessment* Concurrent CRS No Concurrent CRS Grade 1 Administer tocilizumab per Table 1 for management of Grade 1 CRS.
In addition, administer one dose of dexamethasone 10 mg intravenously.
If not improving after 2 days, repeat dexamethasone 10 mg intravenously.Administer one dose of dexamethasone 10 mg intravenously.
If not improving after 2 days, repeat dexamethasone 10 mg intravenously.Consider levetiracetam for seizure prophylaxis. Grade 2 Administer tocilizumab per Table 1 for management of Grade 2 CRS.
In addition, administer dexamethasone 10 mg intravenously four times a day.
If improving, continue corticosteroids until the severity is Grade 1 or less, then quickly taper as clinically appropriate.
If not improving, manage as appropriate grade below.Administer dexamethasone 10 mg intravenously four times a day.
If improving, continue corticosteroids until the severity is Grade 1 or less, then quickly taper as clinically appropriate.
If not improving, manage as appropriate grade below.Consider levetiracetam for seizure prophylaxis. Grade 3 Administer tocilizumab per Table 1 for management of Grade 2 CRS.
In addition, administer methylprednisolone 1000 mg intravenously once daily.
If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then taper as clinically appropriate.
If not improving, manage as Grade 4.Administer methylprednisolone 1000 mg intravenously once daily.
If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then taper as clinically appropriate.
If not improving, manage as Grade 4.Consider levetiracetam for seizure prophylaxis. Grade 4 Administer tocilizumab per Table 1 for management of Grade 2 CRS.
In addition, administer methylprednisolone 1000 mg intravenously twice per day.
If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then taper as clinically appropriate.
If not improving, consider 1000 mg of methylprednisolone intravenously 3 times a day or alternate therapy.†Administer methylprednisolone 1000 mg intravenously twice per day.
If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then taper as clinically appropriate.
If not improving, consider 1000 mg of methylprednisolone intravenously 3 times a day or alternate therapy.†Consider levetiracetam for seizure prophylaxis. -
3 DOSAGE FORMS AND STRENGTHS
YESCARTA is available as a cell suspension for infusion.
A single dose of YESCARTA contains 2 × 106 CAR-positive viable T cells per kg of body weight (or maximum of 2 × 108 CAR-positive viable T cells for patients 100 kg and above) in approximately 68 mL suspension in an infusion bag [see How Supplied/Storage and Handling (16)].
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome
CRS, including fatal or life-threatening reactions, occurred following treatment with YESCARTA. CRS occurred in 90% (379/422) of patients with non-Hodgkin lymphoma (NHL) receiving YESCARTA, including ≥ Grade 3 (Lee grading system1) CRS in 9%. CRS occurred in 93% (256/276) of patients with large B-cell lymphoma (LBCL), including ≥ Grade 3 CRS in 9% [see Adverse Reactions (6)]. Among patients with LBCL who died after receiving YESCARTA, four had ongoing CRS events at the time of death. For patients with LBCL in ZUMA-1, the median time to onset of CRS was 2 days following infusion (range: 1 to 12 days) and the median duration of CRS was 7 days (range: 2 to 58 days). For patients with LBCL in ZUMA-7, the median time to onset of CRS was 3 days following infusion (range: 1 to 10 days) and the median duration was 7 days (range: 2 to 43 days).
CRS occurred in 84% (123/146) of patients with indolent non-Hodgkin lymphoma (iNHL) in ZUMA-5, including ≥ Grade 3 CRS in 8% [see Adverse Reactions (6)]. Among patients with iNHL who died after receiving YESCARTA, one patient had an ongoing CRS event at the time of death. The median time to onset of CRS was 4 days (range: 1 to 20 days) and the median duration was 6 days (range: 1 to 27 days) for patients with iNHL.
Key manifestations of CRS (≥ 10%) in all patients combined included fever (85%), hypotension (40%), tachycardia (32%), chills (22%), hypoxia (20%), headache (15%), and fatigue (12%). Serious events that may be associated with CRS include cardiac arrhythmias (including atrial fibrillation and ventricular tachycardia), renal insufficiency, cardiac failure, respiratory failure, cardiac arrest, capillary leak syndrome, multi-organ failure, and hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) [see Adverse Reactions (6)].
The impact of tocilizumab and/or corticosteroids on the incidence and severity of CRS was assessed in two subsequent cohorts of LBCL patients in ZUMA-1. Among patients who received tocilizumab and/or corticosteroids for ongoing Grade 1 events (see Table 1) [see Clinical Trials Experience (6.1)], CRS occurred in 93% (38/41), including 2% (1/41) with Grade 3 CRS; no patients experienced a Grade 4 or 5 event. The median time to onset of CRS was 2 days (range: 1 to 8 days) and the median duration of CRS was 7 days (range: 2 to 16 days).
Prophylactic treatment with corticosteroids was administered to a cohort of 39 patients for 3 days beginning on the day of infusion of YESCARTA [see Clinical Trials Experience (6.1)]. Thirty-one of the 39 patients (79%) developed CRS at which point the patients were managed with tocilizumab and/or therapeutic doses of corticosteroids with no patients developing Grade 3 or higher CRS. The median time to onset of CRS was 5 days (range: 1 to 15 days) and the median duration of CRS was 4 days (range: 1 to 10 days). Although there is no known mechanistic explanation, consider the risk and benefits of prophylactic corticosteroids in the context of pre-existing comorbidities for the individual patient and the potential for the risk of Grade 4 and prolonged neurologic toxicities [See Neurologic Toxicities (5.2)].
Ensure that 2 doses of tocilizumab are available prior to infusion of YESCARTA. Monitor patients at least daily for 7 days at the certified healthcare facility following infusion for signs and symptoms of CRS. Monitor patients for signs or symptoms of CRS for 4 weeks after infusion. Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time [see Patient Counseling Information (17)]. At the first sign of CRS, institute treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids as indicated [see Dosage and Administration (2.3)].
5.2 Neurologic Toxicities
Neurologic toxicities (including ICANS) that were fatal or life-threatening occurred following treatment with YESCARTA. Neurologic toxicities occurred in 78% (330/422) of patients with NHL receiving YESCARTA, including ≥ Grade 3 cases in 25%.
Neurologic toxicities occurred in 87% (94/108) of patients with LBCL in ZUMA-1, including ≥ Grade 3 cases in 31% and in 74% (124/168) of patients in ZUMA-7 including ≥ Grade 3 cases in 25%. The median time to onset was 4 days (range: 1 to 43 days) and the median duration was 17 days in patients with LBCL in ZUMA-1. The median time to onset for neurologic toxicity was 5 days (range:1 to 133 days) and median duration was 15 days in patients with LBCL in ZUMA-7. Neurologic toxicities occurred in 77% (112/146) of patients with iNHL, including ≥ Grade 3 in 21%. The median time to onset was 6 days (range: 1 to 79 days) and the median duration was 16 days. Ninety-eight percent of all neurologic toxicities in patients with LBCL and 99% of all neurologic toxicities in patients with iNHL occurred within the first 8 weeks of YESCARTA infusion. Neurologic toxicities occurred within the first 7 days of YESCARTA infusion in 87% of affected patients with LBCL and 74% of affected patients with iNHL.
The most common neurologic toxicities (≥ 10%) in all patients combined included encephalopathy (50%), headache (43%), tremor (29%), dizziness (21%), aphasia (17%), delirium (15%), and insomnia (10%). Prolonged encephalopathy lasting up to 173 days was noted. Serious events including aphasia, leukoencephalopathy, dysarthria, lethargy, and seizures occurred with YESCARTA. Fatal and serious cases of cerebral edema and encephalopathy, including late-onset encephalopathy, have occurred in patients treated with YESCARTA.
The impact of tocilizumab and/or corticosteroids on the incidence and severity of neurologic toxicities was assessed in two subsequent cohorts of LBCL patients in ZUMA-1. Among patients who received corticosteroids at the onset of Grade 1 toxicities (see Table 2), neurologic toxicities occurred in 78% (32/41) and 20% (8/41) had Grade 3 neurologic toxicities; no patients experienced a Grade 4 or 5 event. The median time to onset of neurologic toxicities was 6 days (range: 1 to 93 days) with a median duration of 8 days (range: 1 to 144 days). Prophylactic treatment with corticosteroids was administered to a cohort of 39 patients for 3 days beginning on the day of infusion of YESCARTA [see Clinical Trials Experience (6.1)]. Of these 39 patients, 85% (33/39) developed neurologic toxicities; 8% (3/39) developed Grade 3 and 5% (2/39) developed Grade 4 neurologic toxicities. The median time to onset of neurological toxicities was 6 days (range: 1 to 274 days) with a median duration of 12 days (range: 1 to 107 days). Prophylactic corticosteroids for management of CRS and neurologic toxicities may result in higher grade of neurologic toxicities or prolongation of neurologic toxicities, delay the onset and decrease the duration of CRS [See Cytokine Release Syndrome (5.1)].
Monitor patients at least daily for 7 days at the certified healthcare facility following infusion for signs and symptoms of neurologic toxicities. Monitor patients for signs or symptoms of neurologic toxicities for 4 weeks after infusion and treat promptly [see Dosage and Administration (2.3)].
5.3 YESCARTA and TECARTUS REMS
Because of the risk of CRS and neurologic toxicities, YESCARTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA and TECARTUS REMS [see Boxed Warning and Warnings and Precautions (5.1 and 5.2)]. The required components of the YESCARTA and TECARTUS REMS are:
- Healthcare facilities that dispense and administer YESCARTA must be enrolled and comply with the REMS requirements. Certified healthcare facilities must have on-site, immediate access to tocilizumab, and ensure that a minimum of 2 doses of tocilizumab are available for each patient for infusion within 2 hours after YESCARTA infusion, if needed for treatment of CRS.
Further information is available at www.YescartaTecartusREMS.com or 1-844-454-KITE (5483).
5.4 Hypersensitivity Reactions
Allergic reactions may occur with the infusion of YESCARTA. Serious hypersensitivity reactions, including anaphylaxis, may be due to dimethyl sulfoxide (DMSO) or residual gentamicin in YESCARTA.
5.5 Serious Infections
Severe or life-threatening infections occurred in patients after YESCARTA infusion. Infections (all grades) occurred in 45% of patients with NHL. Grade 3 or higher infections occurred in 17% of patients, including Grade 3 or higher infections with an unspecified pathogen in 12%, bacterial infections in 5%, viral infections in 3%, and fungal infections in 1%. YESCARTA should not be administered to patients with clinically significant active systemic infections. Monitor patients for signs and symptoms of infection before and after YESCARTA infusion and treat appropriately. Administer prophylactic antimicrobials according to local guidelines.
Febrile neutropenia was observed in 36% of patients with NHL after YESCARTA infusion and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad-spectrum antibiotics, fluids, and other supportive care as medically indicated.
In immunosuppressed patients, including those who have received YESCARTA, life-threatening and fatal opportunistic infections including disseminated fungal infections (e.g., candida sepsis and aspergillus infections) and viral reactivation (e.g., human herpes virus-6 [HHV-6] encephalitis and JC virus progressive multifocal leukoencephalopathy [PML]) have been reported. The possibility of HHV-6 encephalitis and PML should be considered in immunosuppressed patients with neurologic events and appropriate diagnostic evaluations should be performed.
Hepatitis B Virus Reactivation
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, has occurred in patients treated with drugs directed against B cells, including YESCARTA. Perform screening for HBV, HCV, and HIV and management in accordance with clinical guidelines before collection of cells for manufacturing.
5.6 Prolonged Cytopenias
Patients may exhibit cytopenias for several weeks following lymphodepleting chemotherapy and YESCARTA infusion. Grade 3 or higher cytopenias not resolved by Day 30 following YESCARTA infusion occurred in 39% of all patients with NHL and included neutropenia (33%), thrombocytopenia (13%), and anemia (8%). Monitor blood counts after YESCARTA infusion.
5.7 Hypogammaglobulinemia
B-cell aplasia and hypogammaglobulinemia can occur in patients receiving treatment with YESCARTA. Hypogammaglobulinemia was reported as an adverse reaction in 14% of all patients with NHL. Monitor immunoglobulin levels after treatment with YESCARTA and manage using infection precautions, antibiotic prophylaxis, and immunoglobulin replacement.
The safety of immunization with live viral vaccines during or following YESCARTA treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during YESCARTA treatment, and until immune recovery following treatment with YESCARTA.
5.8 Secondary Malignancies
Patients treated with YESCARTA may develop secondary malignancies. T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies, including YESCARTA. Mature T cell malignancies, including CAR-positive tumors, may present as soon as weeks following infusion, and may include fatal outcomes [see Boxed Warning, Adverse Reactions (6.3), Patient Counseling Information (17)].
Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Kite at 1-844-454-KITE (5483) to obtain instructions on patient samples to collect for testing.
5.9 Effects on Ability to Drive and Use Machines
Due to the potential for neurologic events, including altered mental status or seizures, patients receiving YESCARTA are at risk for altered or decreased consciousness or coordination in the 8 weeks following YESCARTA infusion. Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, during this initial period.
-
6 ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling:
- Cytokine Release Syndrome [see Warnings and Precautions (5.1, 5.3)]
- Neurologic Toxicities [see Warnings and Precautions (5.2, 5.3)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
- Serious Infections [see Warnings and Precautions (5.5)]
- Prolonged Cytopenias [see Warnings and Precautions (5.6)]
- Hypogammaglobulinemia [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in the WARNINGS AND PRECAUTIONS reflect exposure to a single dose of YESCARTA in one randomized, open-label study with 168 patients with relapsed or refractory LBCL (ZUMA-7) and two open-label, single-arm studies with 108 patients with relapsed or refractory LBCL (ZUMA-1 study) and 146 patients with relapsed or refractory iNHL (including 124 with FL; ZUMA-5 study).
Relapsed or Refractory Large B-cell Lymphoma
ZUMA-7
The safety of YESCARTA was evaluated in ZUMA-7, a randomized, open-label, multicenter study in which patients with primary refractory LBCL or first relapse of LBCL received YESCARTA (N = 168) or standard therapy (N = 168) [see Clinical Studies (14)]. Patients had not yet received treatment for relapsed or refractory lymphoma and were potential candidates for autologous HSCT. The trial excluded patients who were not deemed candidates for transplant or who had a history of central nervous system (CNS) disorders (such as seizures or cerebrovascular ischemia), serious or uncontrolled infection, or autoimmune disease requiring systemic immunosuppression. The study required ANC ≥ 1000/mm3, platelet count ≥ 75,000/mm3, creatinine clearance ≥ 60 ml/min, AST/ALT ≤ 2.5 × ULN, and total bilirubin ≤ 1.5 mg/dL.
The median age of the YESCARTA-treated safety population was 59 years (range: 21 to 80 years); 62% were male. The baseline Eastern Cooperative Oncology Group (ECOG) performance status was 0 in 54% of patients and 1 in 46%.
The most common non-laboratory adverse reactions to YESCARTA (incidence ≥ 20%) included fever, CRS, fatigue, hypotension, encephalopathy, tachycardia, diarrhea, headache, musculoskeletal pain, nausea, febrile neutropenia, chills, cough, infection with unspecified pathogen, dizziness, tremor, decreased appetite, edema, hypoxia, abdominal pain, aphasia, constipation, and vomiting. Serious adverse reactions occurred in 50% of patients. The most common serious adverse reactions (> 5%) included CRS, fever, encephalopathy, hypotension, infection with unspecified pathogen, and pneumonia. Fatal adverse reactions occurred in 2% of patients.
The most common (≥ 10%) Grade 3 or higher non-laboratory adverse reactions included febrile neutropenia, encephalopathy, and hypotension.
Sixty-seven percent (112/168) of patients received tocilizumab after infusion of YESCARTA.
Table 3 summarizes selected non-laboratory adverse reactions in patients treated with YESCARTA, and Table 4 summarizes selected new or worsening Grade 3 or 4 laboratory abnormalities.
Table 3. Adverse Reactions in ≥ 10% of Patients Treated with YESCARTA in ZUMA-7 Adverse Reaction YESCARTA
N = 168Any Grade (%) Grade 3 or Higher (%) The following events were also counted in the incidence of CRS: coagulopathy, tachycardia, arrhythmia, cardiac failure, diarrhea, nausea, vomiting, fever, fatigue, chills, edema, decreased appetite, musculoskeletal pain, headache, tremor, dizziness, renal insufficiency, cough, hypoxia, dyspnea, pleural effusion, respiratory failure, rash, hypotension, and hypertension. - *
- Tachycardia includes tachycardia, sinus tachycardia.
- †
- Arrhythmia includes arrhythmia, atrial fibrillation, bradycardia, electrocardiogram QT prolonged, extrasystoles, sinus bradycardia, supraventricular extrasystoles, supraventricular tachycardia, ventricular extrasystoles, ventricular tachycardia.
- ‡
- Diarrhea includes diarrhea, colitis.
- §
- Abdominal pain includes abdominal pain, abdominal discomfort, abdominal pain lower, abdominal pain upper, dyspepsia.
- ¶
- Fever includes pyrexia.
- #
- Fatigue includes fatigue, asthenia, malaise.
- Þ
- Edema includes edema, face edema, fluid overload, generalized edema, hypervolemia, localized edema, edema genital, edema peripheral, periorbital edema, peripheral swelling, pulmonary edema.
- ß
- Musculoskeletal pain includes musculoskeletal pain, arthralgia, arthritis, back pain, bone pain, flank pain, groin pain, musculoskeletal chest pain, myalgia, neck pain, non-cardiac chest pain, pain in extremity.
- à
- Motor dysfunction includes muscle contractions involuntary, muscle spasms, muscle twitching, muscular weakness.
- è
- Encephalopathy includes encephalopathy, altered state of consciousness, amnesia, apraxia, bradyphrenia, cognitive disorder, confusional state, depressed level of consciousness, disturbance in attention, dysarthria, dysgraphia, dyspraxia, lethargy, loss of consciousness, memory impairment, mental impairment, mental status changes, metabolic encephalopathy, slow speech, somnolence, toxic encephalopathy.
- ð
- Headache includes headache and tension headache.
- ø
- Dizziness includes dizziness, dizziness postural, presyncope, syncope, vertigo.
- ý
- Neuropathy peripheral includes hypoesthesia, lumbar radiculopathy, neuropathy peripheral, paresthesia, peroneal nerve palsy, sciatica.
- £
- Insomnia includes insomnia and sleep deficit.
- ¥
- Delirium includes delirium, agitation, delusion, disorientation, hallucination, irritability, restlessness.
- Œ
- Renal insufficiency includes acute kidney injury, blood creatinine increased, chronic kidney disease.
- œ
- Cough includes cough, productive cough, upper-airway cough syndrome.
- Ɖ
- Rash includes rash, dermatitis, dermatitis allergic, dermatitis bullous, drug eruption, erythema, pruritus, rash macular, rash maculo-papular, rash pruritic, urticaria.
- A
- Hypotension includes hypotension, capillary leak syndrome, orthostatic hypotension.
Febrile neutropenia 31 31 Cardiac Disorders Tachycardia * 43 2 Arrhythmia † 14 3 Gastrointestinal Disorders Diarrhea ‡ 42 3 Nausea 40 2 Abdominal pain § 20 4 Constipation 20 0 Vomiting 20 0 Dry Mouth 10 0 General Disorders and Administration Site Conditions Fever ¶ 93 9 Fatigue # 52 7 Chills 28 1 Edema Þ 23 1 Immune System Disorders Cytokine release syndrome 92 7 Hypogammaglobulinemia 11 0 Infections and Infestations Infections with pathogen unspecified 25 8 Viral infections 15 4 Bacterial infections 10 5 Fungal infections 10 1 Metabolism and Nutrition Disorders Decreased appetite 24 4 Musculoskeletal and Connective Tissue Disorders Musculoskeletal pain ß 40 1 Motor dysfunction à 15 4 Nervous System Disorders Encephalopathy è 46 18 Headache ð 41 3 Tremor 25 1 Dizziness ø 25 4 Aphasia 20 7 Neuropathy peripheral ý 11 2 Psychiatric Disorders Insomnia £ 13 0 Delirium ¥ 12 4 Renal and Urinary Disorders Renal insufficiency Œ 11 2 Respiratory, Thoracic and Mediastinal Disorders Cough œ 27 1 Hypoxia 21 9 Skin and Subcutaneous Tissue Disorders Rash Ɖ 17 1 Vascular Disorders Hypotension A 47 11 Other clinically important adverse reactions that occurred in less than 10% of patients treated with YESCARTA include the following:
- Blood and lymphatic system disorders: Coagulopathy (9%)
- Cardiac disorders: Cardiac failure (1%)
- Eye Disorders: Visual impairment (7%)
- Infections and infestations: Pneumonia (8%), Sepsis (4%)
- Nervous system disorders: Ataxia (6%), seizure (3%), myoclonus (2%), facial paralysis (2%), paresis (2%)
- Respiratory, thoracic and mediastinal disorders: Dyspnea (8%), pleural effusion (6%), respiratory failure (2%)
- Vascular disorders: Hypertension (9%), thrombosis (7%)
Laboratory abnormalities:
Table 4. Grade 3 or 4 Laboratory Abnormalities Occurring in ≥ 10% of Patients in ZUMA-7 Following Treatment with YESCARTA* (N = 168) Laboratory Abnormality YESCARTA Grades 3 or 4 (%) - *
- Baseline lab values were assessed prior to lymphodepleting chemotherapy.
Leukocyte decrease 95 Neutrophil decrease 94 Lymphocyte decrease 94 Hemoglobin decrease 40 Platelet decrease 26 Sodium decrease 12 Glucose increase 11 ZUMA-1
The safety of YESCARTA was evaluated in ZUMA-1, a study in which 108 patients with relapsed or refractory LBCL received CD19-positive CAR T cells based on a recommended dose which was weight-based [see Clinical Studies (14)]. Patients with a history of CNS disorders (such as seizures or cerebrovascular ischemia) or autoimmune disease requiring systemic immunosuppression were ineligible. The median age of the study population was 58 years (range: 23 to 76 years); 68% were male. The baseline Eastern Cooperative Oncology Group (ECOG) performance status was 0 in 43% of patients and 1 in 57% of patients.
The most common adverse reactions (incidence ≥ 20%) included CRS, fever, hypotension, encephalopathy, tachycardia, fatigue, headache, decreased appetite, chills, diarrhea, febrile neutropenia, infections with pathogen unspecified, nausea, hypoxia, tremor, cough, vomiting, dizziness, constipation, and cardiac arrhythmias. Serious adverse reactions occurred in 52% of patients. The most common serious adverse reactions (> 2%) included encephalopathy, fever, lung infection, febrile neutropenia, cardiac arrhythmia, cardiac failure, urinary tract infection, renal insufficiency, aphasia, cardiac arrest, Clostridium difficile infection, delirium, hypotension, and hypoxia.
The most common (≥ 10%) Grade 3 or higher reactions included febrile neutropenia, fever, CRS, encephalopathy, infections with pathogen unspecified, hypotension, hypoxia, and lung infections.
Forty-five percent (49/108) of patients received tocilizumab after infusion of YESCARTA.
Table 5 summarizes non-laboratory adverse reactions that occurred in ≥ 10% of patients treated with YESCARTA, and Table 6 describes the laboratory abnormalities of Grade 3 or 4 that occurred in ≥ 10% of patients.
Table 5. Adverse Reactions Observed in ≥ 10% of Patients Treated with YESCARTA in ZUMA-1 (N = 108) Adverse Reaction Any Grade (%) Grade 3 or Higher (%) The following events were also counted in the incidence of CRS: tachycardia, arrhythmia, fever, chills, hypoxia, renal insufficiency, and hypotension. - *
- Tachycardia includes tachycardia, sinus tachycardia.
- †
- Arrhythmia includes arrhythmia, atrial fibrillation, atrial flutter, atrioventricular block, bundle branch block right, electrocardiogram QT prolonged, extra-systoles, heart rate irregular, supraventricular extra systoles, supraventricular tachycardia, ventricular arrhythmia, ventricular tachycardia.
- ‡
- Abdominal pain includes abdominal pain, abdominal pain lower, abdominal pain upper.
- §
- Fever includes fever, febrile neutropenia.
- ¶
- Fatigue includes fatigue, malaise.
- #
- Edema includes face edema, generalized edema, local swelling, localized edema, edema, edema genital, edema peripheral, periorbital edema, peripheral swelling, scrotal edema.
- Þ
- Hypogammaglobulinemia includes hypogammaglobulinemia, blood immunoglobulin D decreased, blood immunoglobulin G decreased.
- ß
- Motor dysfunction includes muscle spasms, muscular weakness.
- à
- Pain in extremity includes pain not otherwise specified, pain in extremity.
- è
- Encephalopathy includes cognitive disorder, confusional state, depressed level of consciousness, disturbance in attention, encephalopathy, hypersomnia, leukoencephalopathy, memory impairment, mental status changes, paranoia, somnolence, stupor.
- ð
- Headache includes headache, head discomfort, sinus headache, procedural headache.
- ø
- Dizziness includes dizziness, presyncope, syncope.
- ý
- Aphasia includes aphasia, dysphasia.
- £
- Delirium includes agitation, delirium, delusion, disorientation, hallucination, hyperactivity, irritability, restlessness.
- ¥
- Hypoxia includes hypoxia, oxygen saturation decreased.
- Œ
- Cough includes cough, productive cough, upper-airway cough syndrome.
- œ
- Dyspnea includes acute respiratory failure, dyspnea, orthopnea, respiratory distress.
- Ɖ
- Hypotension includes diastolic hypotension, hypotension, orthostatic hypotension.
- A
- Thrombosis includes deep vein thrombosis, embolism, embolism venous, pulmonary embolism, splenic infarction, splenic vein thrombosis, subclavian vein thrombosis, thrombosis, thrombosis in device.
Blood and Lymphatic System Disorders Febrile neutropenia 34 31 Cardiac Disorders Tachycardia * 57 2 Arrhythmia † 23 7 Gastrointestinal Disorders Diarrhea 38 4 Nausea 34 0 Vomiting 26 1 Constipation 23 0 Abdominal pain ‡ 14 1 Dry mouth 11 0 General Disorders and Administration Site Conditions Fever § 86 16 Fatigue ¶ 46 3 Chills 40 0 Edema # 19 1 Immune System Disorders Cytokine release syndrome 94 13 Hypogammaglobulinemia Þ 15 0 Infections and Infestations Infections with pathogen unspecified 26 16 Viral infections 16 4 Bacterial infections 13 9 Investigations Decreased appetite 44 2 Weight decreased 16 0 Dehydration 11 3 Musculoskeletal and Connective Tissue Disorders Motor dysfunction ß 19 1 Pain in extremity à 17 2 Back pain 15 1 Muscle pain 14 1 Arthralgia 10 0 Nervous System Disorders Encephalopathy è 57 29 Headache ð 45 1 Tremor 31 2 Dizziness ø 21 1 Aphasia ý 18 6 Psychiatric Disorders Delirium £ 17 6 Respiratory, Thoracic and Mediastinal Disorders Hypoxia ¥ 32 11 Cough Œ 30 0 Dyspnea œ 19 3 Pleural effusion 13 2 Renal and Urinary Disorders Renal insufficiency 12 5 Vascular Disorders Hypotension Ɖ 57 15 Hypertension 15 6 Thrombosis A 10 1 Other clinically important adverse reactions that occurred in less than 10% of patients treated with YESCARTA include the following:
- Blood and lymphatic system disorders: Coagulopathy (2%)
- Cardiac disorders: Cardiac failure (6%), cardiac arrest (4%)
- Immune system disorders: Hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) (1%), hypersensitivity (1%)
- Infections and infestations disorders: Fungal infections (5%)
- Nervous system disorders: Ataxia (6%), seizure (4%), dyscalculia (2%), myoclonus (2%)
- Respiratory, thoracic and mediastinal disorders: Pulmonary edema (9%)
- Skin and subcutaneous tissue disorders: Rash (9%)
- Vascular disorders: Capillary leak syndrome (3%)
Laboratory abnormalities:
Table 6. Grade 3 or 4 Laboratory Abnormalities Occurring in ≥ 10% of Patients in ZUMA-1 Following Treatment with YESCARTA* (N = 108) Laboratory Abnormality Grades 3 or 4 (%) - *
- Baseline lab values were assessed prior to lymphodepleting chemotherapy.
Lymphocyte decrease 96 Leukocyte decrease 96 Neutrophil decrease 92 Hemoglobin decrease 60 Platelet decrease 56 Phosphate decrease 52 Sodium decrease 19 Albumin decrease 19 Direct bilirubin increased 14 Uric acid increased 13 Potassium decrease 11 The safety and efficacy of YESCARTA was evaluated in two subsequent cohorts of LBCL patients. The first subsequent, open label, safety management cohort in ZUMA-1 evaluated the safety and efficacy of YESCARTA with the use of tocilizumab and/or corticosteroid and prophylactic levetiracetam (750 mg PO or IV twice daily) for Grade 1 CRS or neurologic events (see Tables 1 and 2). A total of 46 patients with relapsed or refractory LBCL were enrolled and 41 patients were treated with YESCARTA. Of the remaining 5 patients who were not treated, 2 patients died prior to receiving YESCARTA and 3 patients were ineligible due to disease progression. Twenty-eight patients (68%) treated with YESCARTA received bridging therapy between leukapheresis and lymphodepleting chemotherapy. Thirty-two patients (78%) treated with YESCARTA received tocilizumab and/or corticosteroid for CRS and/or neurologic events. Fifteen of 36 with Grade 1 CRS and 21 of 24 patients with Grade 2 CRS received tocilizumab and/or corticosteroids. Among patients who received treatment for Grade 1 or Grade 2 CRS, most patients (13 of 15 and 19 of 21 patients, respectively) received both tocilizumab and corticosteroids. Most patients received 1 or 2 doses of each drug. Ten of 27 patients with Grade 1 and 7 of 15 patients with Grade 2 neurologic events received corticosteroids alone or in combination with tocilizumab.
The second subsequent, open label, safety management cohort in ZUMA-1 evaluated the safety and efficacy of YESCARTA with the use of prophylactic corticosteroids (oral dexamethasone 10 mg once daily for 3 days, starting prior to YESCARTA infusion on Day 0) and prophylactic levetiracetam (750 mg PO or IV) [see Warnings and Precautions (5.1 and 5.2)].
Relapsed or Refractory Follicular Lymphoma
The safety of YESCARTA was evaluated in ZUMA-5, a study that included 146 patients with relapsed or refractory iNHL (124 patients with FL and 22 with marginal zone lymphoma) who received CD19-positive CAR T cells [see Clinical Studies (14)]. Patients with a history of CNS disorders or autoimmune disease requiring systemic immunosuppression were ineligible. The median age was 61 years (range: 34 to 79 years), 43% were female, 93% were white, 3% were black, and 1% were Asian.
The most common non-laboratory adverse reactions (incidence ≥ 20%) included fever, CRS, hypotension, encephalopathy, fatigue, headache, infections with pathogen unspecified, tachycardia, febrile neutropenia, musculoskeletal pain, nausea, tremor, chills, diarrhea, constipation, decreased appetite, cough, vomiting, hypoxia, arrhythmia, and dizziness. Serious adverse reactions occurred in 48% of patients. Serious adverse reactions in > 2% of patients included febrile neutropenia, encephalopathy, fever, CRS, infections with pathogen unspecified, pneumonia, hypoxia, and hypotension.
The most common (≥ 10%) Grade 3 or higher reactions included febrile neutropenia, encephalopathy, and infections with pathogen unspecified. Fatal adverse reactions occurred in 1% of patients and included CRS and fungal infection.
Fifty-one percent (75/146) of patients received tocilizumab after infusion of YESCARTA.
Table 7 summarizes the adverse reactions, excluding laboratory terms, that occurred in at least 10% of patients treated with YESCARTA and Table 8 describes Grade 3 or 4 laboratory abnormalities that developed or worsened in at least 10% of patients.
Table 7. Adverse Reactions in ≥ 10% of Patients Treated with YESCARTA in ZUMA-5 (N = 146) Adverse Reaction Any Grade (%) Grade 3 or Higher (%) - *
- Febrile neutropenia includes febrile neutropenia, fever overlapping with neutropenia.
- †
- Tachycardia includes tachycardia, sinus tachycardia.
- ‡
- Arrhythmia includes atrial fibrillation, atrioventricular block first degree, bradycardia, sinus bradycardia, supraventricular tachycardia, ventricular arrhythmia, ventricular extra systoles, ventricular tachycardia, electrocardiogram QT prolonged, electrocardiogram T wave inversion.
- §
- Diarrhea includes diarrhea, colitis, enteritis.
- ¶
- Abdominal pain includes abdominal pain, abdominal discomfort, abdominal pain lower, abdominal pain upper, abdominal tenderness, dyspepsia, epigastric discomfort.
- #
- Fatigue includes asthenia, fatigue, decreased activity, malaise.
- Þ
- Edema includes edema, face edema, generalized edema, localized edema, edema peripheral, peripheral swelling, pulmonary edema, swelling face.
- ß
- Immunoglobulins decreased includes hypogammaglobulinemia, blood immunoglobulin G decreased.
- à
- Pneumonia includes pneumonia streptococcal, pneumonia, lung infiltration. Pneumonia is also summarized under infections with pathogen unspecified.
- è
- Decreased appetite includes decreased appetite, hypophagia.
- ð
- Musculoskeletal pain includes musculoskeletal pain, arthralgia, back pain, bone pain, flank pain, groin pain, musculoskeletal chest pain, myalgia, neck pain, osteoarthritis, pain in extremity.
- ø
- Motor dysfunction includes motor dysfunction, muscle rigidity, muscle spasms, muscle strain, muscular weakness.
- ý
- Encephalopathy includes agraphia, amnesia, aphonia, apraxia, CAR T-cell-related encephalopathy syndrome, cognitive disorder, disturbance in attention, dysarthria, dysgraphia, dyskinesia, encephalopathy, lethargy, loss of consciousness, memory impairment, somnolence, speech disorder, confusional state, mental status changes, immune effector cell-associated neurotoxicity, neurotoxicity, toxic encephalopathy.
- £
- Dizziness includes dizziness, presyncope, syncope, vertigo.
- ¥
- Neuropathy peripheral includes allodynia, cervical radiculopathy, hyperesthesia, hypoesthesia, neuralgia, neuropathy peripheral, paresthesia, peripheral sensory neuropathy.
- Œ
- Ataxia includes ataxia, balance disorder, gait disturbance, vestibular disorder.
- œ
- Delirium includes agitation, delirium, hallucination, restlessness.
- Ɖ
- Affective disorder includes anxiety, depression, impulsive behavior, mania, panic attack.
- A
- Cough includes cough, productive cough, upper-airway cough syndrome.
- B
- Dyspnea includes dyspnea, dyspnea exertional.
- C
- Rash includes dermatitis bullous, erythema, pruritus, rash, rash macular, rash maculo-papular, Stevens-Johnson syndrome, urticaria.
- D
- Hypotension includes capillary leak syndrome, hypotension, hypoperfusion, orthostatic hypotension.
- E
- Thrombosis includes deep vein thrombosis, embolism, peripheral ischemia, pulmonary embolism, thrombosis in device, vascular occlusion, jugular vein thrombosis.
Blood and lymphatic system disorders Febrile neutropenia * 41 41 Cardiac Disorders Tachycardia † 44 1 Arrhythmia ‡ 21 2 Gastrointestinal Disorders Nausea 40 0 Diarrhea § 29 1 Constipation 28 0 Vomiting 24 1 Abdominal pain ¶ 16 0 General Disorders and Administration Site Conditions Fever 85 8 Fatigue # 49 1 Chills 29 0 Edema Þ 13 1 Immune System Disorders Cytokine release syndrome 84 8 Immunoglobulins decreased ß 18 1 Infections and Infestations Infections with pathogen unspecified 45 14 Pneumonia à 13 8 Fungal infections 12 2 Viral Infections 13 2 Metabolism and Nutrition Disorders Decreased appetite è 26 1 Musculoskeletal and Connective Tissue Disorders Musculoskeletal pain ð 40 1 Motor dysfunction ø 18 2 Nervous System Disorders Encephalopathy ý 49 16 Headache 45 1 Tremor 31 1 Dizziness £ 20 0 Aphasia 14 4 Neuropathy peripheral ¥ 12 0 Ataxia Œ 10 0 Psychiatric Disorders Delirium œ 16 5 Insomnia 16 0 Affective disorder Ɖ 10 1 Respiratory, Thoracic and Mediastinal Disorders Cough A 25 0 Hypoxia 23 8 Dyspnea B 12 1 Nasal congestion 10 0 Skin and Subcutaneous Tissue Disorders Rash C 19 3 Vascular Disorders Hypotension D 51 4 Hypertension 13 6 Thrombosis E 12 4 Other clinically important adverse reactions that occurred in less than 10% of patients treated with YESCARTA include the following:
- Blood and lymphatic system disorders: Coagulopathy (6%)
- Cardiac disorders: Cardiac failure (2%)
- Eye disorders: Visual impairment (5%), blindness (1%)
- Gastrointestinal disorders: Dysphagia (6%)
- General disorders and administration site conditions: Multiple organ dysfunction syndrome (1%)
- Infections and infestations: Bacterial infections (8%), sepsis (2%), herpesvirus infection (4%)
- Musculoskeletal and connective tissue disorders: Muscle injury (1%)
- Nervous system disorders: Seizure (2%), hemiparesis (2%), ischemic stroke (1%)
- Renal and urinary disorders: Renal insufficiency (8%)
- Respiratory, thoracic and mediastinal disorders: Respiratory failure (1%)
- Vascular disorders: Hemorrhage (8%)
Laboratory abnormalities:
Table 8. Grade 3 or 4 Laboratory Abnormalities Occurring in ≥ 10% of Patients in ZUMA-5 Following Treatment with YESCARTA* (N = 146) Laboratory Abnormality Grades 3 or 4 (%) - *
- Baseline lab values were assessed prior to lymphodepleting chemotherapy.
Lymphocyte decrease 96 Leukocyte decrease 94 Neutrophil decrease 92 Platelet decrease 35 Hemoglobin decrease 32 Phosphate decrease 25 Sodium decrease 10 Glucose increase 10 Calcium decrease 10 6.2 Immunogenicity
YESCARTA has the potential to induce anti-product antibodies. The immunogenicity of YESCARTA has been evaluated using an enzyme-linked immunosorbent assay (ELISA) for the detection of binding antibodies against FMC63, the originating antibody of the anti-CD19 CAR. Eleven patients (4%) tested positive for pre-dose anti-FMC63 antibodies at baseline in ZUMA-7 and ZUMA-1, and one patient (1%) who had a negative test result at baseline had a positive test result post administration of YESCARTA in the screening ELISA in ZUMA-7. In ZUMA-5, 19 patients (13%) were antibody-positive at baseline, and 3 patients (2%) who had negative test results at baseline had positive test results post administration of YESCARTA in the screening ELISA. Results of a confirmatory cell-based assay, leveraging a properly folded and expressed extracellular portion of the CAR (ScFv, hinge and linker) demonstrated that all patients treated with YESCARTA that had a positive result in the screening ELISA were antibody negative at all time points tested. There is no evidence that the kinetics of initial expansion and persistence of YESCARTA, or the safety or effectiveness of YESCARTA, was altered in these patients.
6.3 Postmarketing Experience
Because adverse events to marketed products are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to product exposure.
The following adverse event has been identified during postmarketing use of YESCARTA.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data with YESCARTA use in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with YESCARTA to assess whether it can cause fetal harm when administered to a pregnant woman. It is not known if YESCARTA has the potential to be transferred to the fetus. Based on the mechanism of action, if the transduced cells cross the placenta, they may cause fetal toxicity, including B-cell lymphocytopenia. Therefore, YESCARTA is not recommended for women who are pregnant, and pregnancy after YESCARTA infusion should be discussed with the treating physician.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% – 4% and 15% – 20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of YESCARTA in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for YESCARTA and any potential adverse effects on the breastfed infant from YESCARTA or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy status of females with reproductive potential should be verified. Sexually active females of reproductive potential should have a pregnancy test prior to starting treatment with YESCARTA.
Contraception
See the prescribing information for fludarabine and cyclophosphamide for information on the need for effective contraception in patients who receive the lymphodepleting chemotherapy.
There are insufficient exposure data to provide a recommendation concerning duration of contraception following treatment with YESCARTA.
-
11 DESCRIPTION
YESCARTA (axicabtagene ciloleucel) is a CD19-directed genetically modified autologous T cell immunotherapy. To prepare YESCARTA, a patient's own T cells are harvested and genetically modified ex vivo by retroviral transduction to express a chimeric antigen receptor (CAR) comprising a murine anti-CD19 single chain variable fragment (scFv) linked to CD28 and CD3-zeta co-stimulatory domains. The anti-CD19 CAR T cells are expanded and infused back into the patient, where they can recognize and eliminate CD19-expressing target cells.
YESCARTA is prepared from the patient's peripheral blood mononuclear cells, which are obtained via a standard leukapheresis procedure. The mononuclear cells are enriched for T cells and activated with anti-CD3 antibody in the presence of IL-2, then transduced with the replication incompetent retroviral vector containing the anti-CD19 CAR transgene. The transduced T cells are expanded in cell culture, washed, formulated into a suspension, and cryopreserved. The product must pass a sterility test before release for shipping as a frozen suspension in a patient-specific infusion bag. The product is thawed prior to infusion [see Dosage and Administration (2.2), How Supplied/Storage and Handling (16)].
In addition to T cells, YESCARTA may contain NK and NK-T cells. The formulation contains 5% dimethyl sulfoxide (DMSO) and 2.5% albumin (human).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
YESCARTA, a CD19-directed genetically modified autologous T cell immunotherapy, binds to CD19-expressing cancer cells and normal B cells. Studies demonstrated that following anti-CD19 CAR T cell engagement with CD19-expressing target cells, the CD28 and CD3-zeta co-stimulatory domains activate downstream signaling cascades that lead to T cell activation, proliferation, acquisition of effector functions and secretion of inflammatory cytokines and chemokines. This sequence of events leads to killing of CD19-expressing cells.
12.2 Pharmacodynamics
After YESCARTA infusion, pharmacodynamic responses were evaluated over a 4-week interval by measuring transient elevation of cytokines, chemokines and other molecules in blood. Levels of cytokines and chemokines such as IL-6, IL-8, IL-10, IL-15, TNF-α, IFN-γ, and sIL2Rα were analyzed. Peak elevation was observed within the first 14 days after infusion, and levels generally returned to baseline within 28 days.
Due to the on-target effect of YESCARTA, a period of B-cell aplasia is expected.
Large B-cell lymphoma
Among patients with LBCL with an ongoing response at 24 months in the ZUMA-7 study, 21 of 61 evaluable patients (34%) had no detectable B cells at baseline, and the majority of patients at Month 3 (43 of 69 evaluable patients [62%]) and Month 6 (8 of 13 evaluable patients [62%]) had no detectable B cells. At Month 24, 20 of 24 evaluable patients (83%) had detectable B cells.
Among patients with LBCL with an ongoing response at 24 months in the ZUMA-1 study, 13 of 29 evaluable patients (45%) had no detectable B cells at baseline, and the majority of patients at Month 3 (28 of 35 evaluable patients [80%]) and Month 6 (25 of 32 evaluable patients [78%]) had no detectable B cells. At Month 24, 24 of 32 evaluable patients (75%) had detectable B cells.
12.3 Pharmacokinetics
Following infusion of YESCARTA, anti-CD19 CAR T cells exhibited an initial rapid expansion followed by a decline to near baseline levels by 3 months. Peak levels of anti-CD19 CAR T cells occurred within the first 7 - 14 days after YESCARTA infusion.
Age (range: 21 to 80 years) and gender had no significant impact on AUC Day 0 - 28 and Cmax of YESCARTA.
Large B-cell Lymphoma
Among patients with LBCL in the ZUMA-1 study (n=96 evaluable), the number of anti-CD19 CAR T cells in blood was positively associated with objective response (CR or PR). The median anti-CD19 CAR T cell Cmax levels in responders (n=73) were 205% higher compared to the corresponding level in nonresponders (n=23) (43.6 cells/μL vs 21.2 cells/μL). Median AUC Day 0 - 28 in responding patients (n=73) was 251% of the corresponding level in nonresponders (n=23) (557.1 days × cells/μL vs. 222.0 days × cells/μL).
Among patients with LBCL in the ZUMA-7 study (n=162 evaluable), the number of anti-CD19 CAR T cells in blood was positively associated with objective response [complete remission (CR) or partial remission (PR)]. The median anti-CD19 CAR T cell Cmax levels in responders (n=142) were 275% higher compared to the corresponding level in nonresponders (n=20) (28.9 cells/μL vs 10.5 cells/μL). Median AUC Day 0 - 28 in responding patients (n=142) was 418% of the corresponding level in nonresponders (n=20) (292.9 days × cells/μL vs. 70.1 days × cells/μL). No association between peak anti-CD19 CAR T-cell levels and OS was observed among 163 subjects with an evaluable pharmacokinetic sample when anti-CD19 CAR T-cell peaks were categorized as > median relative to ≤ median.
Follicular Lymphoma
Among patients with FL in the ZUMA-5 study (n=81 evaluable), the median anti-CD19 CAR T cell Cmax levels in responders (n=74) were 40.1 cells/μL and 46.0 cells/μL in nonresponders (n=7). The median AUC Day 0 - 28 in responding FL patients (n=74) were 465.8 days × cells/μL and 404.5 days × cells/μL in nonresponders (n=7).
Some patients required tocilizumab and corticosteroids for management of CRS and neurologic toxicities. Patients treated with tocilizumab (n=44) had 262% and 232% higher anti-CD19 CAR T cells as measured by AUC Day 0 - 28 and Cmax respectively, as compared to patients who did not receive tocilizumab (n=57). Similarly, patients that received corticosteroids (n=26) had 217% and 155% higher AUC Day 0 - 28 and Cmax compared to patients who did not receive corticosteroids (n=75).
Hepatic and renal impairment studies of YESCARTA were not conducted.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Relapsed or Refractory Large B-Cell Lymphoma
ZUMA-7
A randomized, open-label, multicenter trial evaluated the efficacy of YESCARTA in adult patients with relapsed or refractory LBCL after first-line chemoimmunotherapy that included rituximab and anthracycline (ZUMA-7; NCT03391466). Patients had not yet received treatment for relapsed or refractory lymphoma and were potential candidates for autologous HSCT. Patients were required to have primary refractory disease or relapse within 12 months following completion of first-line therapy. The study excluded patients with primary mediastinal B-cell lymphoma, any history of central nervous system lymphoma, need for urgent therapy due to tumor mass effect, active or serious infections, and ECOG performance status of 2 or greater.
In total, 359 patients were randomized in a 1:1 ratio to receive a single infusion of YESCARTA or to receive second-line standard therapy, consisting of 2 or 3 cycles of chemoimmunotherapy followed by high-dose therapy and autologous HSCT in patients who attained CR or PR. Randomization was stratified by response to first-line therapy and second-line age-adjusted International Prognostic Index.
Following lymphodepleting chemotherapy, YESCARTA was administered as a single intravenous infusion at a target dose of 2 × 106 CAR-positive viable T cells/kg (maximum permitted dose: 2 × 108 cells). The lymphodepleting regimen consisted of cyclophosphamide 500 mg/m2 intravenously and fludarabine 30 mg/m2 intravenously, both given on the fifth, fourth, and third day before YESCARTA. All patients who received YESCARTA were monitored at a healthcare facility for a minimum of 7 days. Bridging therapy, administered between leukapheresis and lymphodepleting chemotherapy, was limited to corticosteroids and was permitted for patients with high disease burden.
In the overall study population, the median age was 59 years (range: 21 to 81 years), 66% were male, 83% were white, 6% were Asian, and 5% were Black. The diagnoses included de novo DLBCL NOS (63%), HGBL with or without MYC and BCL-2 and/or BCL-6 rearrangements (19%), and large cell transformation of follicular lymphoma (13%). In total, 74% of patients had primary refractory LBCL, and 26% had relapsed disease within 12 months of first-line therapy.
Of the 180 patients randomized to receive YESCARTA, 178 underwent leukapheresis and 170 were treated with YESCARTA, of whom 60 (33%) received bridging corticosteroid therapy. Eight patients (4%) were not treated following leukapheresis, primarily due to progressive disease, serious adverse events, or death. The median time from leukapheresis to product delivery was 18 days (range: 13 to 49 days), and from leukapheresis to YESCARTA infusion was 26 days (range: 16 to 52 days). The median dose was 2.0 × 106 CAR-positive viable T cells/kg (range: 1.0 to 2.1 × 106 cells/kg).
Of the 179 patients randomized to receive standard therapy, 168 patients received any study treatment, and 62 (35%) received high-dose therapy and on-protocol HSCT. The most common reason for not receiving HSCT was lack of response to salvage chemotherapy.
The primary efficacy measure was event-free survival (EFS) as determined by an independent review committee. Efficacy is summarized in Table 9 and Figure 1. With an estimated median follow-up of 22.1 months overall, the estimated EFS rate at 18 months was 41.5% [95% CI: 34.2, 48.6] in the YESCARTA arm and 17.0% [95% CI: 11.8, 23.0] in the standard therapy arm.
In the YESCARTA arm, the estimated median DOR was 28.4 months (95% CI: 26.9, NE) in patients who achieved CR and 1.6 months (95% CI: 1.4, 1.9) in patients who achieved a best response of PR.
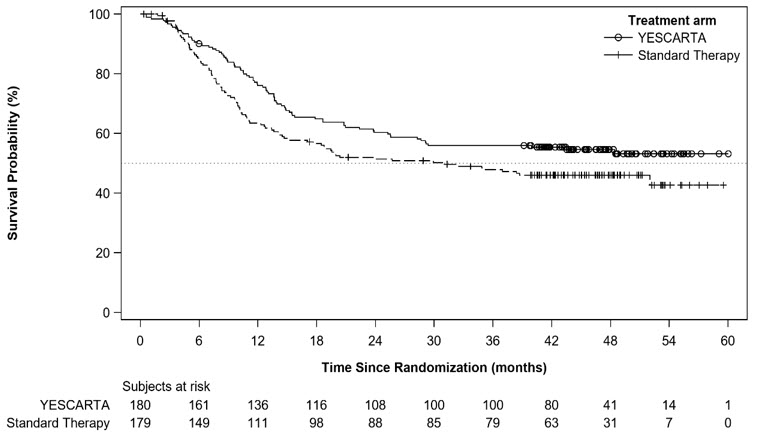
At the time of the primary EFS analysis, the interim analysis of overall survival (OS) did not meet criteria for statistical significance. With an estimated median follow-up of 46.7 months overall, the primary analysis of OS showed a statistically significant improvement in the YESCARTA arm compared to the standard therapy arm. Fifty-seven percent of patients received cellular immunotherapy after no response to or relapse after randomization to standard therapy arm.
The efficacy results are summarized in Table 9 and Figure 1 and Figure 2.
Table 9. Efficacy Results for ZUMA-7 Outcome* YESCARTA
(N = 180)†Standard Therapy
(N = 179)CI, confidence interval; NE, not estimable. - *
- Per the International Working Group Lugano Classification (Cheson 2014), as assessed by the independent review committee.
- †
- Two recipients of non-conformal product are included in the efficacy analysis.
- ‡
- EFS is defined as time from randomization to the earliest date of disease progression or relapse, best response of stable disease up to and including the Day 150 assessment, commencement of new lymphoma therapy, or death from any cause.
- §
- Kaplan-Meier estimate.
- ¶
- Overall survival was conducted at the time of the primary OS analysis
- #
- p-value is compared with 0.0249, the one-sided efficacy boundary (significance level) for the primary OS analysis.
- Þ
- Per Cochran-Mantel-Haenszel method. For all stratified analyses, stratification was based on response to first-line therapy (primary refractory, vs relapse within 6 months of first-line therapy vs relapse within > 6 but ≤ 12 months) and second-line age-adjusted International Prognostic Index.
Event-Free Survival‡ Number of events, n (%) 108 (60) 144 (80) Median, months [95% CI]§ 8.3 [4.5, 15.8] 2.0 [1.6, 2.8] Stratified hazard ratio [95% CI] 0.40 [0.31, 0.51] Stratified log-rank p-value <0.0001 Overall Survival¶ Number of events, n (%) 82 (46) 95 (53) Median OS, months [95% CI]§ NE (28.6, NE) 31.1 (17.1, NE) Stratified hazard ratio [95% CI] 0.73 (0.54, 0.98) Stratified log-rank p-value# 0.0168 Best Objective Response Rate, % [95% CI] 83 [77, 88] 50 [43, 58] Difference in ORR, % [95% CI] 33 [23, 42] Stratified p-valueÞ <0.0001 Complete remission rate, % [95% CI] 65 [58, 72] 32 [26, 40] Partial remission rate, % [95% CI] 18 [13, 25] 18 [13, 24] Progression-Free Survival Number of events, n (%) 93 (52) 81 (45) Median, months [95% CI] § 14.9 [7.2, NE] 5.0 [3.4, 8.5] Stratified hazard ratio [95% CI] 0.56 [0.41, 0.76] Figure 1. Kaplan-Meier Curve of Event-Free Survival in ZUMA-7 (Primary EFS Analysis)
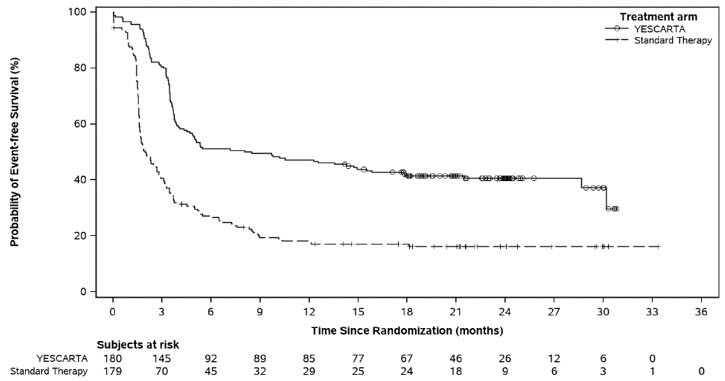
Figure 2. Kaplan-Meier Curve of Overall Survival in ZUMA-7 (Primary OS Analysis)
ZUMA-1
A single-arm, open-label, multicenter trial evaluated the efficacy of a single infusion of YESCARTA in adult patients with relapsed or refractory aggressive B-cell non-Hodgkin lymphoma (ZUMA-1; NCT02348216). Eligible patients had refractory disease to the most recent therapy or relapse within 1 year after autologous hematopoietic stem cell transplantation (HSCT). The study excluded patients with prior allogeneic HSCT, any history of central nervous system lymphoma, ECOG performance status of 2 or greater, absolute lymphocyte count less than 100/µL, creatinine clearance less than 60 mL/min, hepatic transaminases more than 2.5 times the upper limit of normal, cardiac ejection fraction less than 50%, or active serious infection.
Following lymphodepleting chemotherapy, YESCARTA was administered as a single intravenous infusion at a target dose of 2 × 106 CAR-positive viable T cells/kg (maximum permitted dose: 2 × 108 cells). The lymphodepleting regimen consisted of cyclophosphamide 500 mg/m2 intravenously and fludarabine 30 mg/m2 intravenously, both given on the fifth, fourth, and third day before YESCARTA. Bridging chemotherapy between leukapheresis and lymphodepleting chemotherapy was not permitted. All patients were hospitalized for YESCARTA infusion and for a minimum of 7 days afterward.
Of 111 patients who underwent leukapheresis, 101 received YESCARTA. Of the patients treated, the median age was 58 years (range: 23 to 76 years), 67% were male, and 89% were white. Most (76%) had DLBCL, 16% had transformed follicular lymphoma, and 8% had primary mediastinal large B-cell lymphoma. The median number of prior therapies was 3 (range: 1 to 10), 77% of the patients had refractory disease to a second or greater line of therapy, and 21% had relapsed within 1 year of autologous HSCT.
One out of 111 patients did not receive the product due to manufacturing failure. Nine other patients were not treated, primarily due to progressive disease or serious adverse reactions following leukapheresis. The median time from leukapheresis to product delivery was 17 days (range: 14 to 51 days), and the median time from leukapheresis to infusion was 24 days (range: 16 to 73 days). The median dose was 2.0 × 106 CAR-positive viable T cells/kg (range: 1.1 to 2.2 × 106 cells/kg).
Efficacy was established on the basis of complete remission (CR) rate and duration of response (DOR), as determined by an independent review committee (Table 10 and Table 11). The median time to response was 0.9 months (range: 0.8 to 6.2 months). Response durations were longer in patients who achieved CR, as compared to patients with a best response of partial remission (PR) (Table 11). Of the 52 patients who achieved CR, 14 initially had stable disease (7 patients) or PR (7 patients), with a median time to improvement of 2.1 months (range: 1.6 to 5.3 months).
Table 10. Response Rate in Patients with Relapsed or Refractory LBCL in ZUMA-1 Recipients of YESCARTA
(N = 101)CI, confidence interval. - *
- Per 2007 revised International Working Group criteria, as assessed by the independent review committee.
Objective Response Rate*
(95% CI)73 (72%)
(62, 81)Complete Remission Rate
(95% CI)52 (51%)
(41, 62)Partial Remission Rate
(95% CI)21 (21%)
(13, 30)Table 11. Duration of Response in Patients with Relapsed or Refractory LBCL in ZUMA-1 From N of 101 CR, complete remission; DOR, duration of response; NE, not estimable; PR, partial remission. Number of Responders 73 DOR (Months)* Median†
(95% CI)9.2
(5.4, NE)Range‡ 0.03+, 14.4+ DOR if Best Response is CR (Months) Median†
(95% CI)NE
(8.1, NE)Range‡ 0.4, 14.4+ DOR if Best Response is PR (Months) Median†
(95% CI)2.1
(1.3, 5.3)Range‡ 0.03+, 8.4+ Median Follow-up for DOR (Months)*, † 7.9 14.2 Relapsed or Refractory Follicular Lymphoma
Efficacy in FL is based on a single-arm, open-label, multicenter trial (ZUMA-5; NCT03105336) that evaluated a single infusion of YESCARTA in adult patients with relapsed or refractory FL after two or more lines of systemic therapy, including the combination of an anti-CD20 monoclonal antibody and an alkylating agent. The study excluded patients with active or serious infections, transformed lymphoma or other aggressive lymphomas, prior allogeneic HSCT, or any history of CNS lymphoma or CNS disorders. Following lymphodepleting chemotherapy, YESCARTA was administered as a single intravenous infusion with a target dose of 2 × 106 anti-CD19 CAR T cells/kg (maximum permitted dose: 2 × 108 cells). The lymphodepleting regimen consisted of cyclophosphamide 500 mg/m2 intravenously and fludarabine 30 mg/m2 intravenously, both given on the fifth, fourth, and third day before YESCARTA.
Of 123 patients with FL who underwent leukapheresis, 120 received YESCARTA. Of the remaining three patients (2%) who were not treated, one was ineligible due to thrombocytopenia, one went into remission prior to initiating lymphodepletion, and one died of cardiac arrest. There were no manufacturing failures. Of the 120 patients with FL infused with YESCARTA, the 81 consecutive patients included in the primary efficacy analysis had at least 9 months of potential follow-up from date of first response.
Among the 81 patients with FL included in the primary efficacy analysis, the median age was 62 years (range: 34 to 79 years), 46% were female, 93% were white, 4% were black, and 3% were Asian. The median number of prior systemic therapies was 3 (range: 2 to 9), with 32% having 2 prior lines, 22% having 3 prior lines, and 46% having ≥ 4 prior lines. Thirty-one percent had received a PI3K inhibitor, 72% had progression within 6 months of the most recent regimen, and 56% had progression within 24 months of initiating their first anti-CD20 combination therapy. Between leukapheresis and administration of YESCARTA, one patient (1%) in the primary efficacy analysis received bridging therapy.
Among the 81 patients included in the primary efficacy analysis, the median time from leukapheresis to product delivery was 17 days (range: 13 to 33 days) and leukapheresis to product infusion was 27 days (range: 19 to 250 days). The median dose of YESCARTA was 2.0 × 106 CAR T cells/kg (range 1.3 to 2.1 × 106 CAR T cells/kg). All treated patients received YESCARTA infusion on day 0 and were hospitalized until at least day 7.
Efficacy was established on the basis of objective response rate and DOR as determined by an independent review committee (Table 12 and Table 13). The median time to response in the primary efficacy population was 1.0 month (range: 0.8 to 3.1 months).
Table 12. Response Rate in Patients with Relapsed or Refractory FL in ZUMA-5 Primary Efficacy Analysis
(N = 81)All Leukapheresed Patients
(N = 123)CI, confidence interval. - *
- Per the International Working Group Lugano Classification (Cheson 2014), as assessed by the independent review committee.
- †
- Complete remission required documentation of a negative bone marrow biopsy after treatment, in patients who did not have a negative bone marrow biopsy between their most recent disease progression prior to ZUMA-5 and initiation of lymphodepleting chemotherapy.
Objective Response Rate*, n
(95% CI)74 (91%)
(83, 96)110 (89%)
(83, 94)Complete Remission†, n
(95% CI)49 (60%)
(49, 71)76 (62%)
(53, 70)Partial Remission, n
(95% CI)25 (31%)
(21, 42)34 (28%)
(20, 36)Table 13. Duration of Response in Patients with Relapsed or Refractory FL in ZUMA-5 From N of 81 CR, complete remission; DOR, duration of response; NE, not estimable; PR, partial remission. - *
- Among all responders in the primary efficacy population. DOR is measured from the date of first objective response to the date of progression or death from any cause.
- †
- Kaplan-Meier estimate.
- ‡
- A "+" sign indicates a censored value.
- §
- Measured from the date of first objective response to the date of progression or death.
Number of Responders 74 DOR (Months)* Median†
(95% CI)NE
(20.8, NE)Range‡ 0.0, 25.0+ Rate of Continued Remission*, †, § At 12 months (95% CI), % 76.2 (63.9, 84.7) At 18 months (95% CI), % 74.2 (61.5, 83.2) Median Follow-up for DOR (Months)*, † 14.5 - 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
YESCARTA is supplied in an infusion bag (NDC 71287-119-01) containing approximately 68 mL of frozen suspension of genetically modified autologous T cells in 5% DMSO and 2.5% albumin (human).
Each YESCARTA infusion bag is individually packed in a metal cassette (NDC 71287-119-02). YESCARTA is stored in the vapor phase of liquid nitrogen and supplied in a liquid nitrogen dry shipper.
- Match the identity of the patient with the patient identifiers on the cassette and infusion bag upon receipt.
- Store YESCARTA frozen in the vapor phase of liquid nitrogen (less than or equal to minus 150 °C).
- Thaw before using [see Dosage and Administration (2)].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Ensure that patients understand the risk of manufacturing failure (< 1% in clinical trials). In case of a manufacturing failure, a second manufacturing of YESCARTA may be attempted. In addition, while the patient awaits the product, additional chemotherapy (not the lymphodepletion) may be necessary and may increase the risk of adverse events during the pre-infusion period.
Advise patients to seek immediate attention for any of the following:
- Cytokine Release Syndrome (CRS) - Signs or symptoms associated with CRS, including fever, chills, fatigue, tachycardia, nausea, hypoxia, and hypotension [see Warnings and Precautions (5.1) and Adverse Reactions (6)].
- Neurologic Toxicities - Signs or symptoms associated with neurologic events, including encephalopathy, seizures, changes in level of consciousness, speech disorders, tremors, and confusion [see Warnings and Precautions (5.2) and Adverse Reactions (6)].
- Serious Infections - Signs or symptoms associated with infection [see Warnings and Precautions (5.5) and Adverse Reactions (6)].
- Prolonged Cytopenia - Signs or symptoms associated with bone marrow suppression, including neutropenia, anemia, thrombocytopenia, or febrile neutropenia [see Warnings and Precautions (5.6) and Adverse Reactions (6)].
- Secondary Malignancies - Secondary malignancies, including T cell malignancies, have occurred [see Boxed Warning, Warnings and Precautions (5.8), and Adverse Reactions (6.3)].
Advise patients of the need to:
- Refrain from driving or operating heavy or potentially dangerous machinery after YESCARTA infusion for at least 8 weeks after infusion [see Warnings and Precautions (5.9)].
- Have periodic monitoring of blood counts.
- Contact Kite at 1-844-454-KITE (5483) if they are diagnosed with a secondary malignancy [see Warnings and Precautions (5.8)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
MEDICATION GUIDE
YESCARTA (pronounced yes-kar-ta)
(axicabtagene ciloleucel)This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: June 2024 Read this Medication Guide before you start your YESCARTA treatment. The more you know about your treatment, the more active you can be in your care. Talk with your healthcare provider if you have questions about your health condition or treatment. Reading this Medication Guide does not take the place of talking with your healthcare provider about your treatment. What is the most important information I should know about YESCARTA?
YESCARTA may cause side effects that are life-threatening and can lead to death. Call or see your healthcare provider or get emergency help right away if you get any of the following:- Fever (100.4°F/38°C or higher)
- Difficulty breathing
- Chills or shaking chills
- Confusion
- Dizziness or lightheadedness
- Severe nausea, vomiting, or diarrhea
- Fast or irregular heartbeat
- Severe fatigue or weakness
What is YESCARTA?
YESCARTA is a prescription medicine used to treat two types of non-Hodgkin lymphoma:- large B-cell lymphoma when your first treatment did not work or your cancer returned within a year of first treatment, OR when at least two kinds of treatment have failed to control your cancer.
- follicular lymphoma when at least two kinds of treatment have failed to control your cancer.
Before getting YESCARTA, tell your healthcare provider about all your medical problems, including if you have or have had: - Neurologic problems (such as seizures, stroke, or memory loss)
- Lung or breathing problems
- Heart problems
- Liver problems
- Kidney problems
- A recent or active infection
How will I receive YESCARTA? - Since YESCARTA is made from your own white blood cells, your blood will be collected by a process called "leukapheresis" (loo-kah-fur-ee-sis), which will concentrate your white blood cells.
- Your blood cells will be sent to a manufacturing center to make your YESCARTA.
- Before you get YESCARTA, you will get 3 days of chemotherapy to prepare your body.
- When your YESCARTA is ready, your healthcare provider will give it to you through a catheter placed into your vein (intravenous infusion). The infusion usually takes less than 30 minutes.
- You will be monitored where you received your treatment daily for at least 7 days after the infusion.
- You should plan to stay close to a certified healthcare facility for at least 4 weeks after getting YESCARTA. Your healthcare provider will help you with any side effects that may occur.
- You may be hospitalized for side effects and your healthcare provider will discharge you if your side effects are under control, and it is safe for you to leave the hospital.
- Your healthcare provider will want to do blood tests to follow your progress. It is important that you do have your blood tested. If you miss an appointment, call your healthcare provider as soon as possible to reschedule.
- Do not drive, operate heavy machinery, or do other dangerous things for 8 weeks after you get YESCARTA because the treatment can cause sleepiness, confusion, weakness, and temporary memory and coordination problems.
- Do not donate blood, organs, tissues, or cells for transplantation.
What are the possible or reasonably likely side effects of YESCARTA?
The most common side effects of YESCARTA include:- Fever (100.4°F/38°C or higher)
- Low white blood cells (can occur with a fever)
- Low red blood cells
- Low blood pressure (dizziness or lightheadedness, headache, feeling tired, short of breath)
- Fast heartbeat
- Confusion
- Difficulty speaking or slurred speech
- Nausea
- Diarrhea
These are not all the possible side effects of YESCARTA. Call your healthcare provider about any side effects that concern you. You may report side effects to the FDA at 1-800-FDA-1088.General information about the safe and effective use of YESCARTA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. If you would like more information about YESCARTA, talk with your healthcare provider. You can ask your healthcare provider for information about YESCARTA that is written for health professionals. You can get additional information by contacting Kite at 1-844-454-KITE (5483) or at www.Yescarta.com.What are the ingredients in YESCARTA?
Active ingredients: axicabtagene ciloleucel.
Inactive ingredients: albumin (human); DMSO.
YESCARTA is a trademark of Kite Pharma, Inc. All other trademarks referenced herein are the property of their respective owners.
© 2024 Kite Pharma, Inc. All Rights Reserved. - Principal Display Panel - Patient ID Label
-
Package/Label Principal Display Panel
axicabtagene ciloleucel
YESCARTA
STOP
Confirm patient ID prior to infusion
VERIFY PATIENT ID
Lot: 123456789-0X
Kite Patient ID: 123456789
Expiration Date: 31-Dec-2900
First Name M.I.: FIRST NAME W
Last Name: LAST NAME
DOB.: 31-Dec-1900
Hospital Patient ID: 1234567890123456
DIN:
AS-00829 W0123 45 678900
- Principal Display Panel - Lot Label
-
Principal Display Panel - 68 mL Bag Cassette Label
NDC 71287-119-02
axicabtagene ciloleucel
YESCARTA®RX ONLY
FOR AUTOLOGOUS & INTRAVENOUS USE ONLY
No U.S. standard of potencyDose: One sterile bag for infusion.
Contents: Maximum of 2 x 108 autologous anti-CD19 CAR T cells in
approximately 68 mL suspension containing 5% DMSO USP.Gently mix the contents of
the bag while thawingSee package insert for full
prescribing information and
instructions for administrationShip and store in vapor phase
of liquid nitrogen ≤ -150°CDO NOT USE A
LEUKODEPLETING FILTER
DO NOT IRRADIATEManufactured with gentamicin
Not evaluated for infectious
substancesPreservative free
Manufacturer: Kite Pharma, Inc., 2400 Broadway, Santa Monica, CA 90404
Phone: 1-844-454-KITE
U.S. Lic. #2064AS-02683

-
Principal Display Panel - 68 mL Bag Label
NDC 71287-119-01
axicabtagene ciloleucel
YESCARTA®RX ONLY
FOR AUTOLOGOUS & INTRAVENOUS USE ONLY
No U.S. standard of potencyDose: One sterile bag for infusion.
Contents: Maximum of 2 x 108 autologous anti-CD19 CAR T cells in
approximately 68 mL suspension containing 5% DMSO USP.Gently mix the contents of
the bag while thawingSee package insert for full
prescribing information and
instructions for administrationShip and store in vapor phase
of liquid nitrogen ≤ -150°CDO NOT USE A
LEUKODEPLETING FILTER
DO NOT IRRADIATEManufactured with gentamicin
Not evaluated for infectious
substancesPreservative free
Manufacturer: Kite Pharma, Inc., 2400 Broadway, Santa Monica, CA 90404
Phone: 1-844-454-KITE
U.S. Lic. #2064AS-02684

-
INGREDIENTS AND APPEARANCE
YESCARTA
axicabtagene ciloleucel suspensionProduct Information Product Type CELLULAR THERAPY Item Code (Source) NDC:71287-119 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AXICABTAGENE CILOLEUCEL (UNII: U2I8T43Y7R) (AXICABTAGENE CILOLEUCEL - UNII:U2I8T43Y7R) AXICABTAGENE CILOLEUCEL 2000000 in 68 mL Inactive Ingredients Ingredient Name Strength DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71287-119-02 1 in 1 PACKAGE 1 NDC:71287-119-01 68 mL in 1 BAG; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125643 10/18/2017 Labeler - Kite Pharma, Inc. (963353359) Establishment Name Address ID/FEI Business Operations Kite Pharma, Inc. 963353359 MANUFACTURE(71287-119) , LABEL(71287-119) Establishment Name Address ID/FEI Business Operations Kite Pharma, Inc. 116931311 ANALYSIS(71287-119)