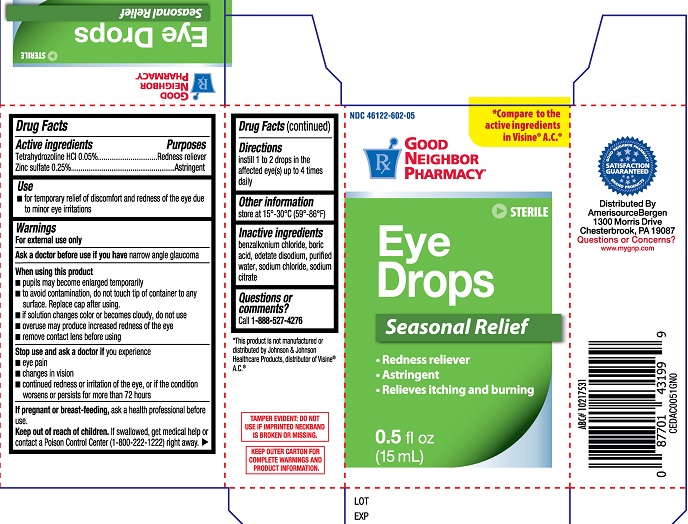
Label: GNP EYE DROPS SEASONAL RELIEF- tetrahydrozoline hcl, zinc sulfate solution
- NDC Code(s): 46122-602-05
- Packager: AmerisourceBergen
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
When using this product
- pupils may become enlarged temporarily
- to avoid contamination, do not touch tip of container to any surface. Replace cap after using
- if solution changes color or becomes cloudy, do not use
- overuse may produce increased redness of the eye
- remove contact lens before using
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GNP EYE DROPS SEASONAL RELIEF
tetrahydrozoline hcl, zinc sulfate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46122-602 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TETRAHYDROZOLINE HYDROCHLORIDE (UNII: 0YZT43HS7D) (TETRAHYDROZOLINE - UNII:S9U025Y077) TETRAHYDROZOLINE HYDROCHLORIDE 0.05 g in 100 mL ZINC SULFATE (UNII: 89DS0H96TB) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 0.25 g in 100 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) WATER (UNII: 059QF0KO0R) BORIC ACID (UNII: R57ZHV85D4) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CITRATE (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46122-602-05 1 in 1 CARTON 08/28/2019 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 08/28/2019 Labeler - AmerisourceBergen (007914906) Registrant - KC Pharmaceuticals, Inc. (174450460) Establishment Name Address ID/FEI Business Operations K.C. Pharmaceuticals, Inc. 174450460 manufacture(46122-602) , pack(46122-602) , label(46122-602)