Label: SOLUVIT N- thiamine mononitrate, riboflavin 5-phosphate sodium anhydrous, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, sodium pantothenate, biotin, sodium ascorbate powder, for solution
- NDC Code(s): 65219-075-25, 65219-077-15
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated June 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Presentation
-
ACTIVE INGREDIENT
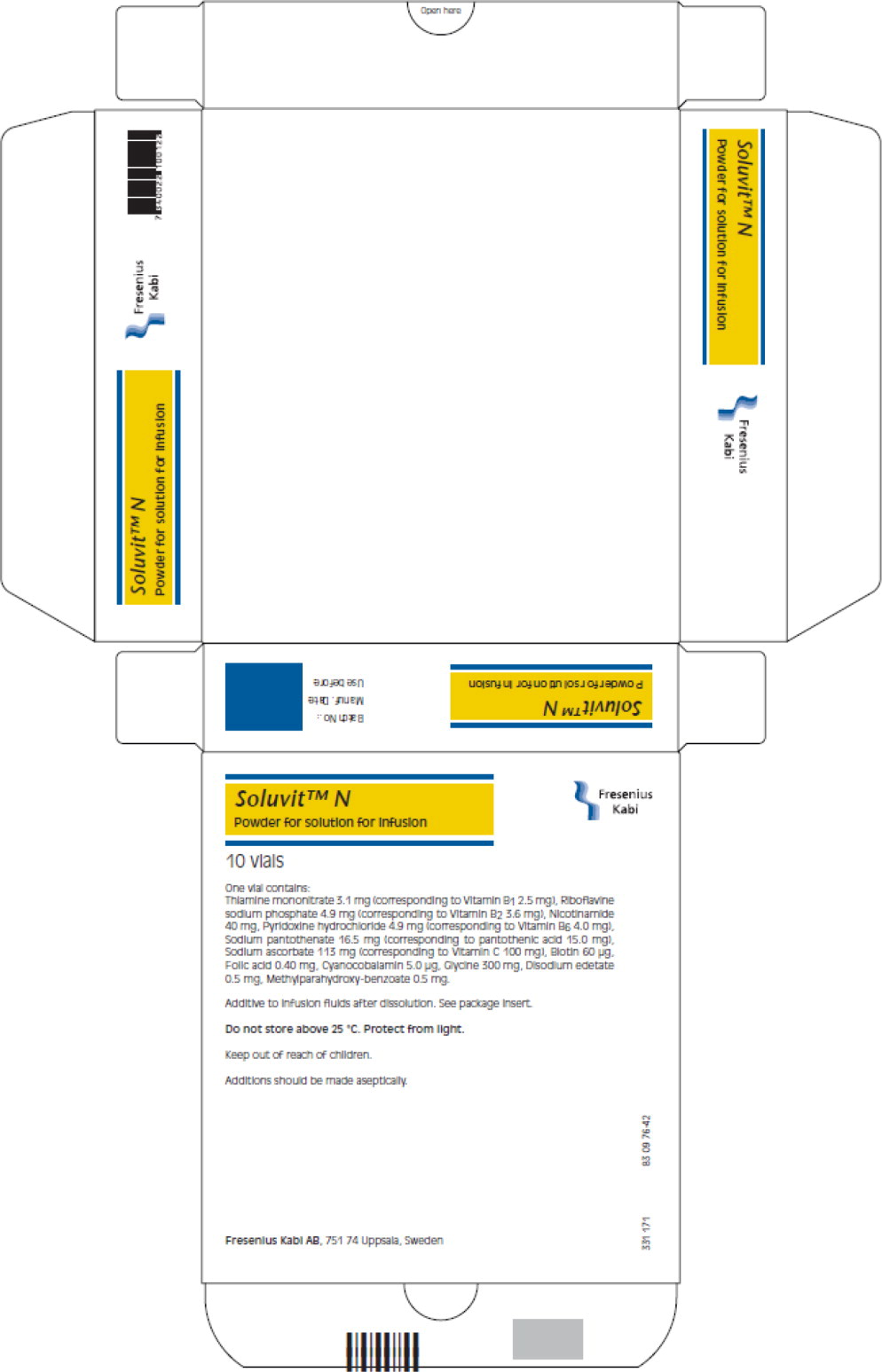
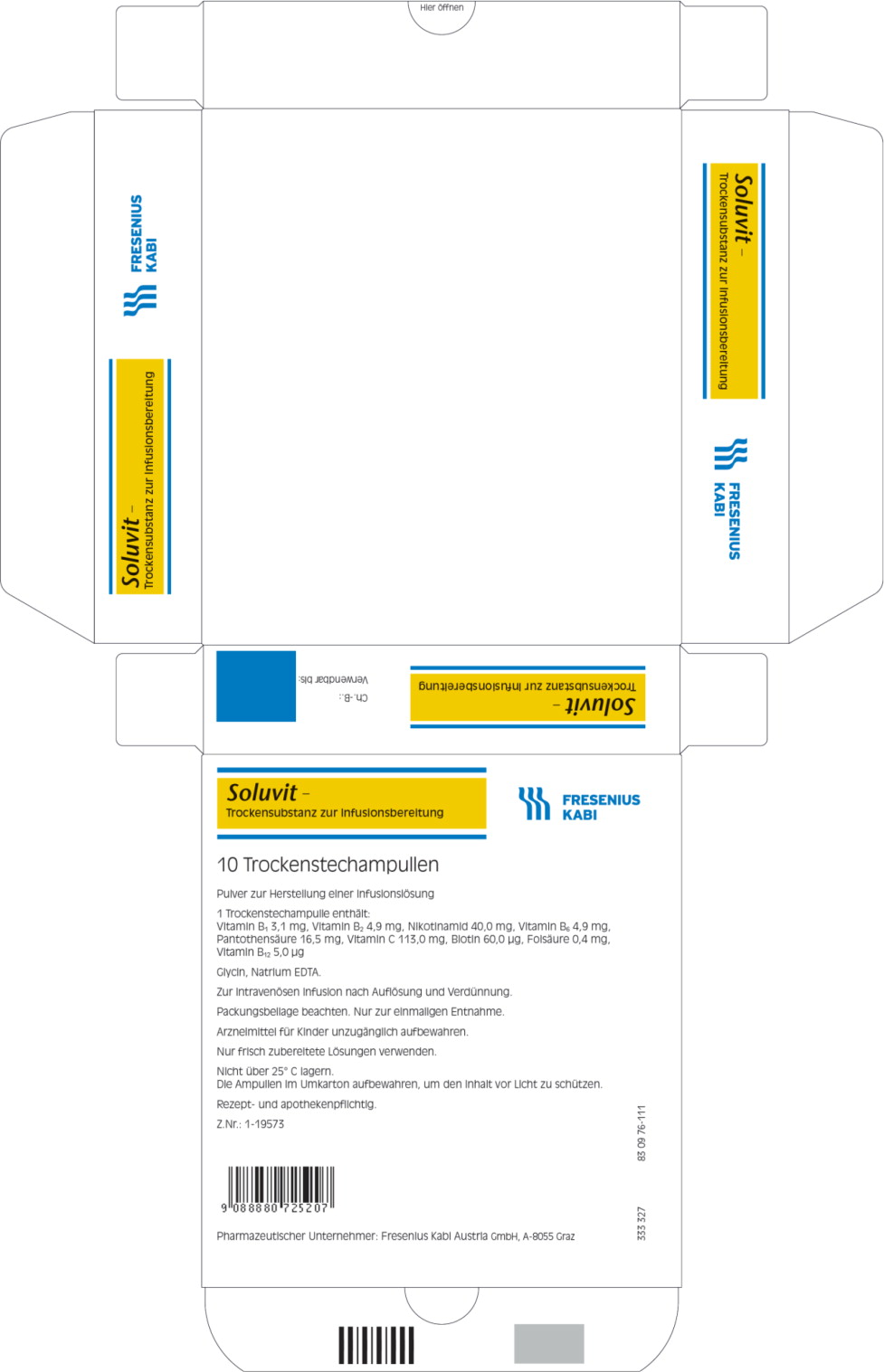
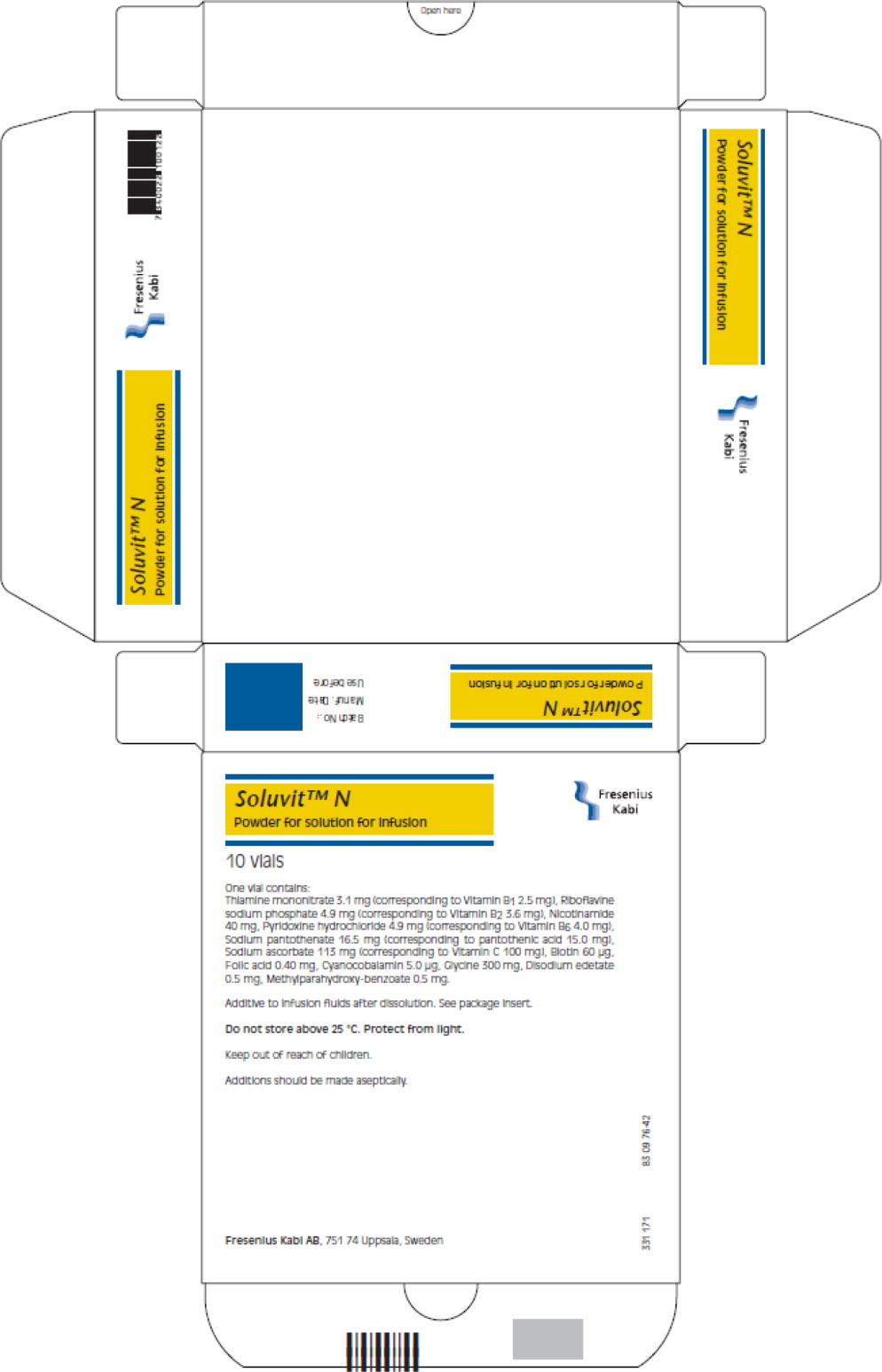
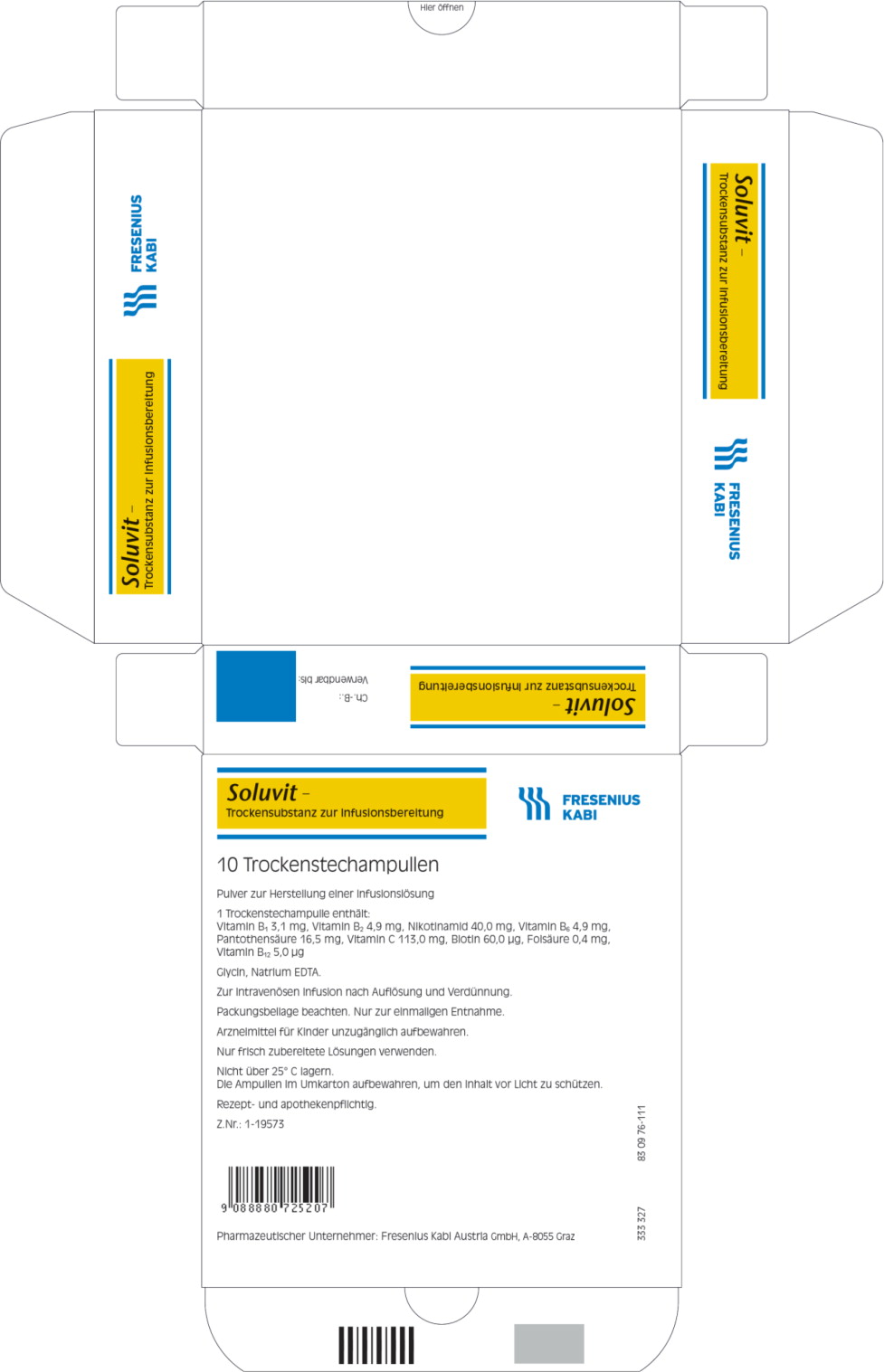
One vial of Soluvit N contains:
Active ingredients Quantity 1 ml of reconstituted Thiamine Soluvit N contains: mononitrate (Corresponding to Vitamin B1 2.5 mg)
Riboflavine sodium3.1 mg 0.31 mg phosphate (Corresponding to
Vitamin B2 3.6 mg)4.9 mg 0.49 mg Nicotinamide
Pyridoxine40 mg 4.0 mg hydrochloride (corresponding to Vitamin B6 4.0 mg)
Sodium4.9 mg 0.49 mg pantothenate (corresponding to pantothenic acid
15.0 mg)16.5 mg 1.65 mg
Sodium ascorbate
(corresponding to
Vitamin C 100 mg)
113 mg
11.3 mgBiotin 60 μg 6.0 μg Folic acid 0.40 mg 40 μg Cyanocobalamin 5.0 μg 0.5 μg For the full list of excipients, see section list of excipients.
- Osmolality in 10 ml of water: approx. 490 mosm/kg water
- pH in 10 ml of water: 5.8
Therapeutic indications
Soluvit N is indicated in adult patients and children as a supplement in intravenous nutrition to meet the daily requirements of water soluble vitamins.
- Posology and method of administration
- Contraindications
-
Special warning and special precaution for use
Soluvit N must not be given undiluted.
When Soluvit N is diluted with water based solutions, the admixture should be protected from light. This is not necessary if Soluvit N is diluted with Intralipid because of the protective effect of the fat emulsion.
Interference with clinical laboratory tests
Biotin may interfere with laboratory tests that are based on a biotin/streptavidin interaction, leading to either falsely decreased or falsely increased test results, depending on the assay. The risk of interference is higher in children and patients with renal impairment and increases with higher doses. When interpreting results of laboratory tests, possible biotin interference has to be taken into consideration, especially if a lack of coherence with the clinical presentation is observed (e.g. thyroid test results mimicking Graves' disease in asymptomatic patients taking biotin or false negative troponin test results in patients with myocardial infarction taking biotin). Alternative tests not susceptible to biotin interference should be used, if available, in cases where interference is suspected. The laboratory personnel should be consulted when ordering laboratory tests in patients taking biotin.
- Interaction with other medicinal products and other forms of interaction
- Fertility, pregnancy and lactation
- Effects on ability to drive and use machines
-
Undesirable effects
Allergic reactions including severe (anaphylactic) reactions may occur in patients hypersensitive to any component of the preparation, e.g. folic acid, thiamine or methyl parahydroxybensoate (frequency not known).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system.
-
Overdose
No adverse effects of an overdose of water soluble vitamins have been reported, with exception of cases of extremly high parenteral doses. Overdoses caused by parenteral preparations for nutritional supplement of water soluble vitamins have not been reported.
No specific treatment is needed. See also section Contraindications.
- List of excipients
- Incompatibilities
-
Shelf-life of the medicinal product as packed for sale
18 months
Shelf-life after mixing
Chemical and physical in-use stability after dilution has been demonstrated for 24 hours at 25°C. From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2-8°C, unless mixing has taken place in controlled and validated aseptic conditions.
- Special precautions for storage
-
Instructions for use/handling, and disposal
Adults and children age 11 years and above:
The contents of one vial of Soluvit N are dissolved by adding 10 ml of:
1. Vitalipid N Adult
or 2. Intralipid 10%, Intralipid 20%, Intralipid 30%, Structolipid
or 3. Water for Injections
or 4. Glucose solution for infusion (5%-50%)
Soluvit N may be added to parenteral nutrition admixtures containing carbohydrates, lipids, amino acids, electrolytes and trace elements provided that compatibility and stability have been confirmed.
Children below 11 years of age:
The contents of one vial are dissolved by adding 10 ml of:
1. Vitalipid N Infant (for children above 10 kg/bw)
or 2. Intralipid 10%, Intralipid 20%
or 3. Water for Injections
or 4. Glucose solution for infusion (5%-50%)
Children weighing less than 10 kg should be given 1 ml of the dissolved mixture per kg body weight per day. Children weighing 10 kg or more should be given 10 ml (one vial) per day.
Due to differences in the dosage regimes for Soluvit N and Vitalipid N Infant, the mixture 1 is not recommended for children weighing less than 10 kg.
Soluvit N may be added to parenteral nutrition admixtures containing carbohydrates, lipids, amino acids, electrolytes and trace elements provided that compatibility and stability have been confirmed.
Disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Manufacturer:
Fresenius Kabi AB, Uppsala, Sweden
March 2019
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SOLUVIT N
thiamine mononitrate, riboflavin 5-phosphate sodium anhydrous, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, sodium pantothenate, biotin, sodium ascorbate powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65219-077 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength thiamine mononitrate (UNII: 8K0I04919X) (thiamine ion - UNII:4ABT0J945J) thiamine 1.581 mg in 1 mL riboflavin 5'-phosphate sodium anhydrous (UNII: 957E53WV42) (riboflavin 5'-phosphate - UNII:7N464URE7E) riboflavin 5'-phosphate 2.45 mg in 1 mL niacinamide (UNII: 25X51I8RD4) (niacinamide - UNII:25X51I8RD4) niacinamide 20.0 mg in 1 mL pyridoxine hydrochloride (UNII: 68Y4CF58BV) (pyridoxine - UNII:KV2JZ1BI6Z) pyridoxine hydrochloride 2.45 mg in 1 mL folic acid (UNII: 935E97BOY8) (folic acid - UNII:935E97BOY8) folic acid 0.216 mg in 1 mL cyanocobalamin (UNII: P6YC3EG204) (cyanocobalamin - UNII:P6YC3EG204) cyanocobalamin 2.775 ug in 1 mL sodium pantothenate (UNII: OES0R93F0C) (pantothenic acid - UNII:19F5HK2737) sodium pantothenate 8.25 mg in 1 mL biotin (UNII: 6SO6U10H04) (biotin - UNII:6SO6U10H04) biotin 30.0 ug in 1 mL sodium ascorbate (UNII: S033EH8359) (ascorbic acid - UNII:PQ6CK8PD0R) ascorbic acid 56.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength edetate disodium (UNII: 7FLD91C86K) glycine (UNII: TE7660XO1C) water (UNII: 059QF0KO0R) methylparaben (UNII: A2I8C7HI9T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65219-077-15 10 in 1 CARTON 07/07/2023 1 2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 07/07/2023 SOLUVIT N
thiamine mononitrate, riboflavin 5-phosphate sodium anhydrous, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, sodium pantothenate, biotin, sodium ascorbate powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65219-075 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength thiamine mononitrate (UNII: 8K0I04919X) (thiamine ion - UNII:4ABT0J945J) thiamine 1.581 mg in 1 mL riboflavin 5'-phosphate sodium anhydrous (UNII: 957E53WV42) (riboflavin 5'-phosphate - UNII:7N464URE7E) riboflavin 5'-phosphate 2.45 mg in 1 mL niacinamide (UNII: 25X51I8RD4) (niacinamide - UNII:25X51I8RD4) niacinamide 20.0 mg in 1 mL pyridoxine hydrochloride (UNII: 68Y4CF58BV) (pyridoxine - UNII:KV2JZ1BI6Z) pyridoxine hydrochloride 2.45 mg in 1 mL folic acid (UNII: 935E97BOY8) (folic acid - UNII:935E97BOY8) folic acid 0.216 mg in 1 mL cyanocobalamin (UNII: P6YC3EG204) (cyanocobalamin - UNII:P6YC3EG204) cyanocobalamin 2.775 ug in 1 mL sodium pantothenate (UNII: OES0R93F0C) (pantothenic acid - UNII:19F5HK2737) sodium pantothenate 8.25 mg in 1 mL biotin (UNII: 6SO6U10H04) (biotin - UNII:6SO6U10H04) biotin 30.0 ug in 1 mL sodium ascorbate (UNII: S033EH8359) (ascorbic acid - UNII:PQ6CK8PD0R) ascorbic acid 56.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength edetate disodium (UNII: 7FLD91C86K) glycine (UNII: TE7660XO1C) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65219-075-25 10 in 1 CARTON 07/07/2023 1 2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 07/07/2023 Labeler - Fresenius Kabi USA, LLC (013547657) Establishment Name Address ID/FEI Business Operations Fresenius Kabi USA, LLC 840771732 ANALYSIS(65219-077, 65219-075) , MANUFACTURE(65219-077, 65219-075)