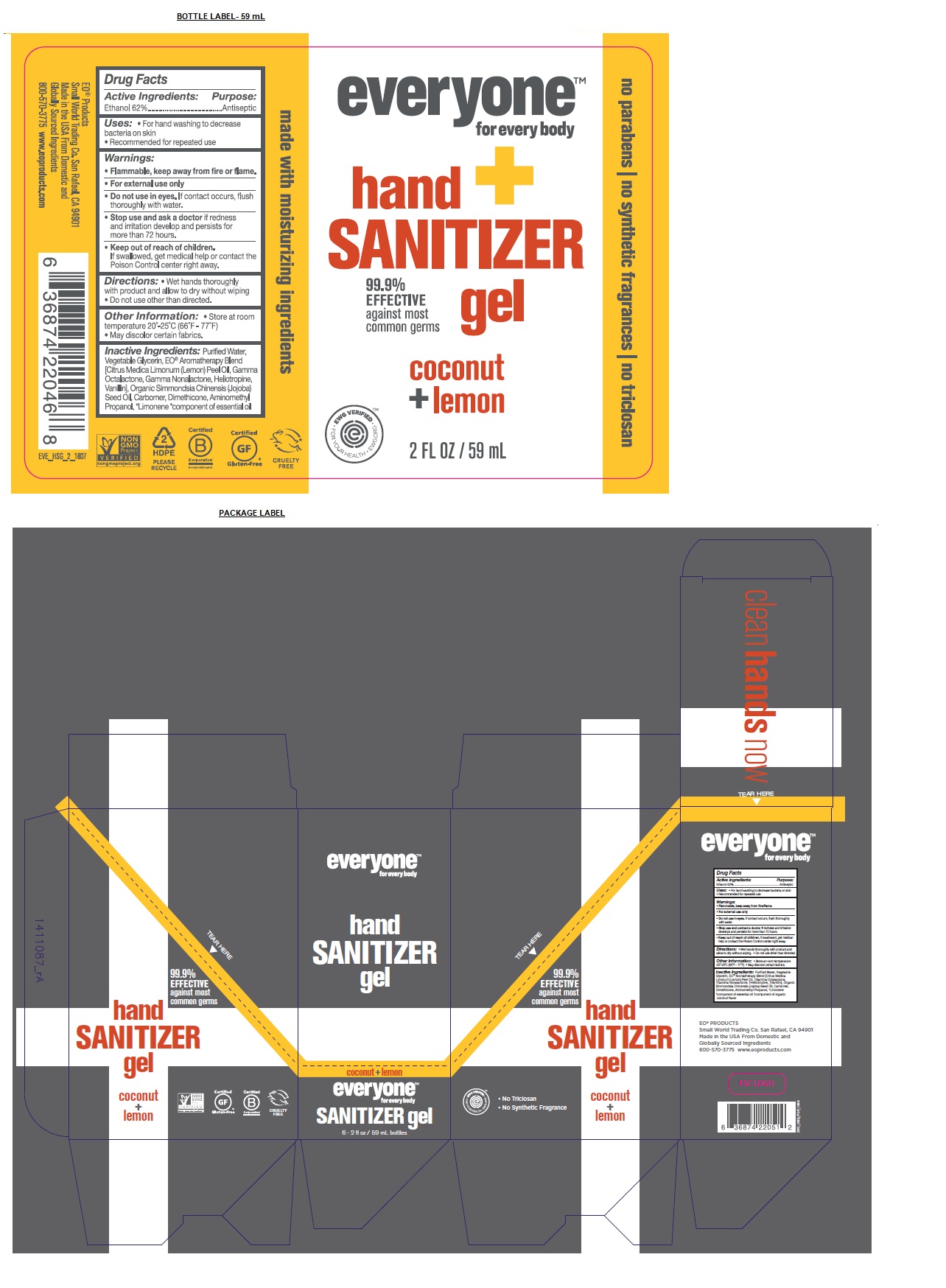
Label: EVERYONE HAND SANITIZER COCONUT LEMON- alcohol gel
- NDC Code(s): 54748-401-02, 54748-401-05, 54748-401-08, 54748-401-09
- Packager: EO Products, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 22, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients:
- Purpose:
- Uses:
- Warnings:
- KEEP OUT OF REACH OF CHILDREN
- Directions:
-
Inactive Ingredients:
Purified Water, Vegetable Glycerin, EO® Aromatherapy Blend [Citrus Medica Limonum (Lemon) Peel Oil, Ethanol, Gamma Octalactone, Gamma Nonalactone, Heliotropine, Vanillin], Organic Simmondsia Chinensis (Jojoba) Seed Oil, Carbomer, Dimethicone, Aminomethyl Propanol, *Limonene
*component of essential oil
- Other Information:
-
SPL UNCLASSIFIED SECTION
99.9% EFFECTIVE against most common germs
- No Disodium EDTA
clean hands now
no parabens / no synthetic fragrances / no triclosan
made with moisturizing ingredients
EO® PRODUCTS
Small World Trading Co. San Rafael, CA 94901
Made in the USA From Domestic and Globally Sourced Ingredients
800-570-3775 www.eoproducts.com
- Packaging
-
INGREDIENTS AND APPEARANCE
EVERYONE HAND SANITIZER COCONUT LEMON
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54748-401 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) LEMON OIL (UNII: I9GRO824LL) .GAMMA.-OCTALACTONE (UNII: UHD6M52X0K) .GAMMA.-NONALACTONE (UNII: I1XGH66S8P) PIPERONAL (UNII: KE109YAK00) VANILLIN (UNII: CHI530446X) JOJOBA OIL (UNII: 724GKU717M) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) DIMETHICONE (UNII: 92RU3N3Y1O) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) LIMONENE, (+)- (UNII: GFD7C86Q1W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54748-401-02 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/07/2013 02/15/2017 2 NDC:54748-401-05 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/31/2018 3 NDC:54748-401-09 6 in 1 PACKAGE 08/31/2018 3 NDC:54748-401-05 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:54748-401-08 1 in 1 CASE 05/15/2015 4 237 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 06/07/2013 Labeler - EO Products, LLC (786611210) Establishment Name Address ID/FEI Business Operations EO Products, LLC 786611210 manufacture(54748-401)