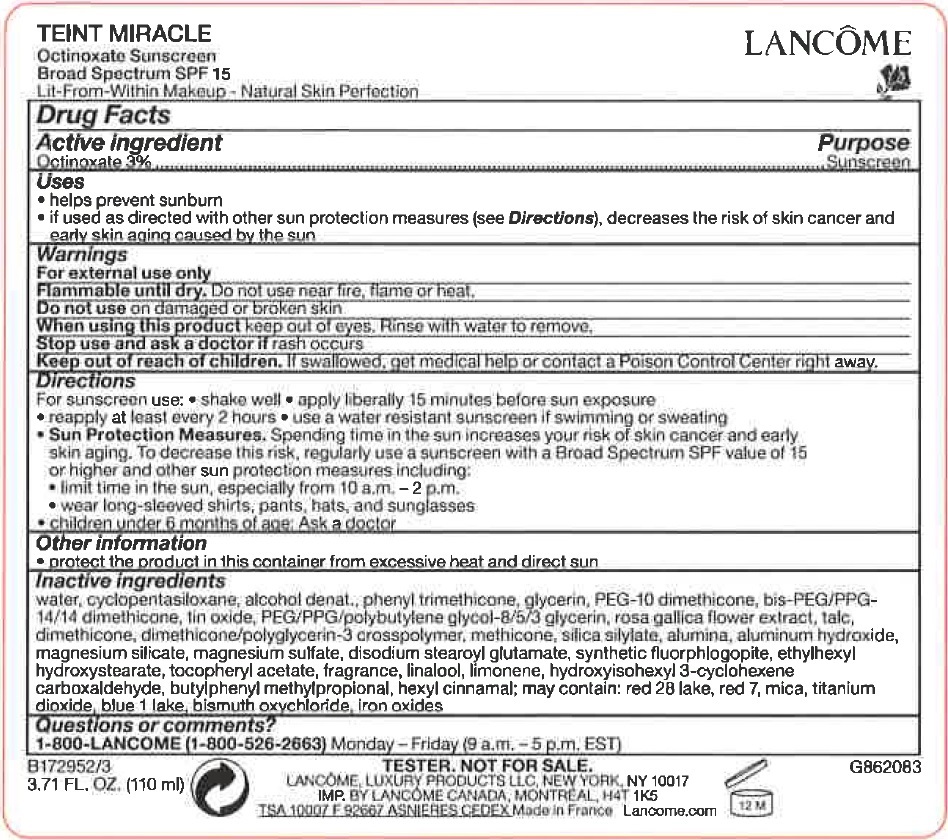
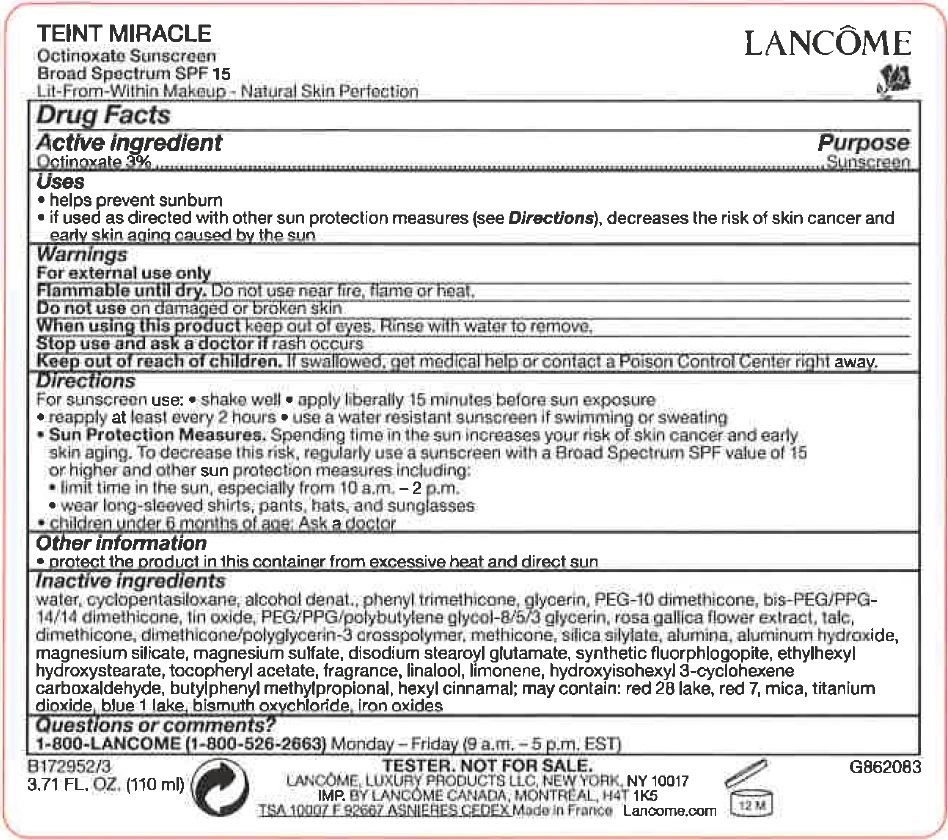
Label: TEINT MIRACLE OCTINOXATE SUNSCREEN BROAD SPECTRUM SPF 15- octinoxate cream
- NDC Code(s): 70581-004-01
- Packager: BPS 60
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 9, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- uses
- Warnings
-
Directions
For sunscreen use: •shake well • apply liberally 15 minutes before sun exposure • reaply at least every 2 hours • use a water resistant sunscreen if swimming or sweating • Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk. regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun. especially from 10 a.m. - 2 p.m. • wear long-sleeved shirts, pants, hats and sunglasses • children under 6 months of age: Ask a doctor
Sun Protection Measures. - Other information
-
Inactive ingredients
water, cyclopentasiloxane, alcohol denat., phenyl trimethicone, glycerin, PEG-10 dimethicone, bis-PEG/PPG-14/14 dimethicone, tin oxide, PEG/PPG/polybutylene glycol-8/5/3 glycerin, rosa gallica flower extract, talc, dimethicone, dimethicone/polyglycerin-3 crosspolymer, methicone, silica silylate, alumina, aluminum hydroxide, magnesium silicate, magnesium sulfate, disodium stearoyl glutamate, synthetic fluorphlogopite, ethylhexyl hydroxystearate, tocopheryl acetate, fragrance, linalool, limonene, hydroxyisohexyl 3-cyclohexene carboxaldehyde, butylphenyl methylpropional, hexyl cinnamal; may contain: red28 lake, red 7, mica, titanium dioxide, blue 1 lake, bismuth oxychloride, Iron oxides
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
TEINT MIRACLE OCTINOXATE SUNSCREEN BROAD SPECTRUM SPF 15
octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70581-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) ETHYLHEXYL HYDROXYSTEARATE (UNII: B7I80BVV5E) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LINALOOL, (+/-)- (UNII: D81QY6I88E) HYDROXYISOHEXYL 3-CYCLOHEXENE CARBOXALDEHYDE (UNII: QUE43B9Z2Q) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ALCOHOL (UNII: 3K9958V90M) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) GLYCERIN (UNII: PDC6A3C0OX) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) STANNIC OXIDE (UNII: KM7N50LOS6) ROSA GALLICA FLOWER (UNII: X8W61WUV70) TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) METHICONE (20 CST) (UNII: 6777U11MKT) ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) MAGNESIUM SILICATE (UNII: 9B9691B2N9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70581-004-01 1 in 1 BOX 04/06/2016 1 110 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/06/2016 Labeler - BPS 60 (272259304) Establishment Name Address ID/FEI Business Operations BPS 60 272259304 pack(70581-004) Establishment Name Address ID/FEI Business Operations SICOS ET CIE 276993581 manufacture(70581-004)