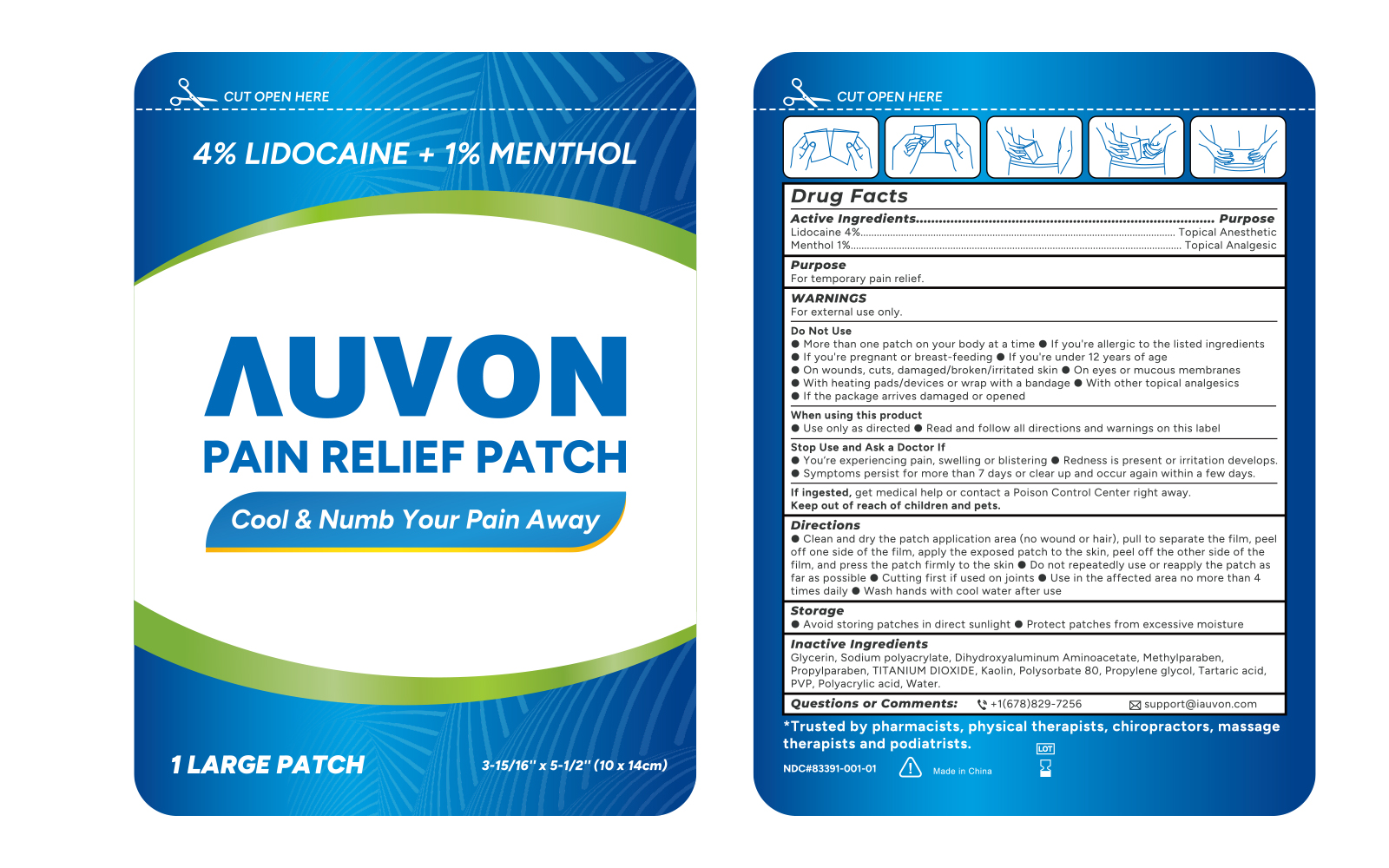
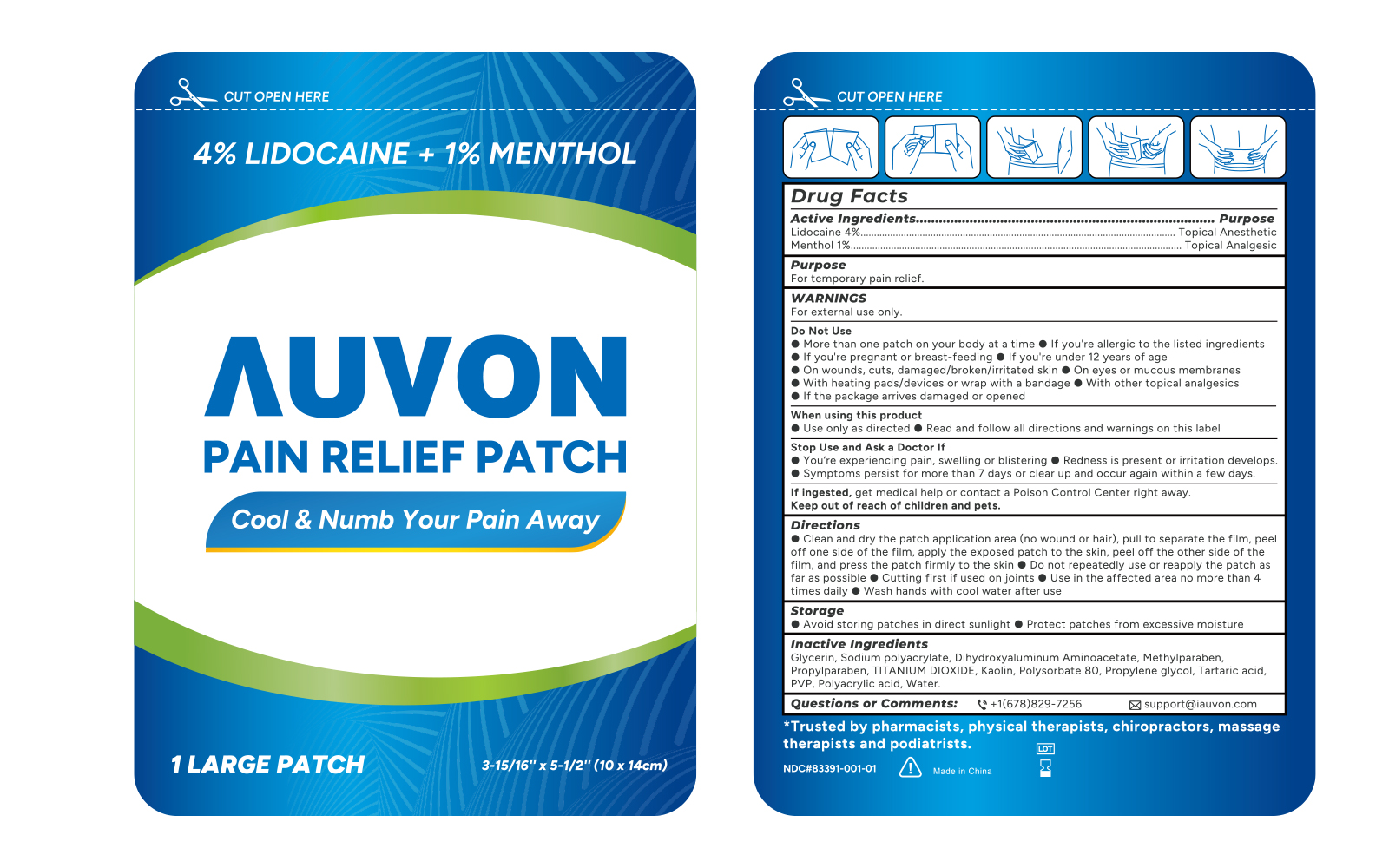
Label: AUVON LIDOCAINE MENTHOL PAIN RELIEF- lidocaine and menthol patch
- NDC Code(s): 83391-001-01, 83391-001-02, 83391-001-03
- Packager: SHENZHEN YUWEN E-COMMERCE CO., LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purposes
- Use
-
Warnings
For external use only
Do not use
- If you are allergic to the listed ingredients
- If you are pregnent or breast feeding
- If you are under 12 years of age
- On wounds, cuts, damaged/broken/irritated skin
- On eyes or mucous membranes
- With heating pads/devices or wrap with a bandage
- With other topical analgesics
- If the package arrives damaged or opened.
When using this product
■ Use only as directed
■ Read and follow all directions and warnings on this label
■ avoid contact with the eyes and mucous membranes
■ rare cases of serious burns have been reported with products of this type
■ a transient burning sensation may occur upon application but generally disappears in several days
■ dispose of used patch in manner that always keeps product away from children and pets. Used patches still
contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
-
Directions
■ Clean and dry the patch application area (no wound or hair), pull to separate the film, peel off one side of the film, apply the exposed patch to the skin, peel off the other side of the film, and press the patch firmly to the skin.
■ Do not repeatedly use or repeatedly reapply the patch as far as possible
■ Cut first if if used on joints
■ Use in te affected area no more than 4 times daily
■ Wash hands with cool water after use
- Storage
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AUVON LIDOCAINE MENTHOL PAIN RELIEF
lidocaine and menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83391-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 370 mg MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 100 mg Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIHYDROXYALUMINUM AMINOACETATE ANHYDROUS (UNII: 1K713C615K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) PROPYLPARABEN (UNII: Z8IX2SC1OH) KAOLIN (UNII: 24H4NWX5CO) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83391-001-01 1 in 1 CARTON; Type 0: Not a Combination Product 04/12/2023 2 NDC:83391-001-02 8 in 1 CARTON; Type 0: Not a Combination Product 04/12/2023 3 NDC:83391-001-03 30 in 1 CARTON; Type 0: Not a Combination Product 04/12/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 04/12/2023 Labeler - SHENZHEN YUWEN E-COMMERCE CO., LTD. (544559614) Registrant - SHENZHEN YUWEN E-COMMERCE CO., LTD. (544559614) Establishment Name Address ID/FEI Business Operations Foshan Aqua Gel Biotech Co., Ltd., 529128763 manufacture(83391-001)