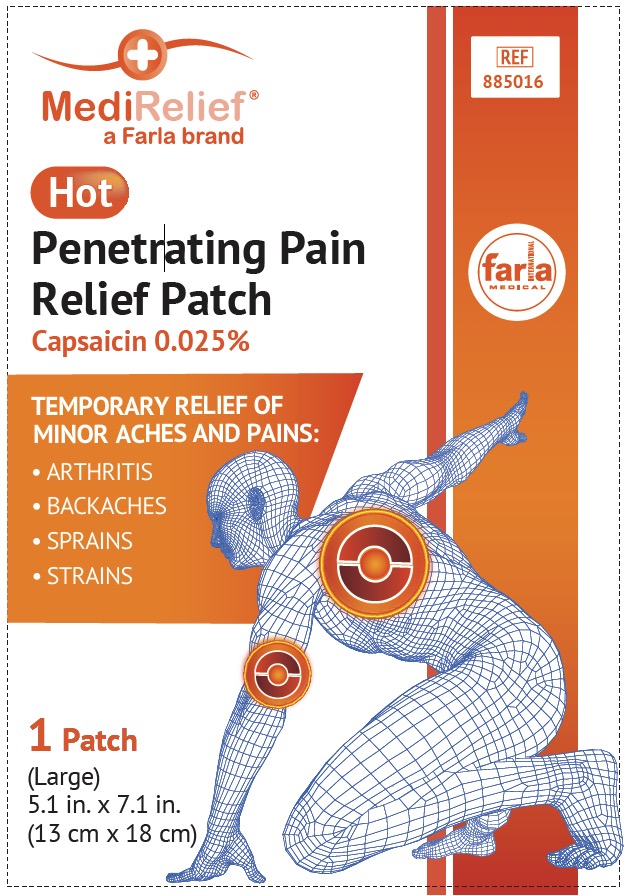
Label: MEDIRELIEF HOT- capsaicin patch
- NDC Code(s): 73486-100-35
- Packager: Farla Medical Healthcare LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with
- simple back aches
- arthritis
- strains
- sprains
Do not use
- on wounds or damaged skin
- on a tight bandage
- with a heating pad
- on sensitive skin
- if allergic to any ingredients in this product
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
KEEP OUT OF REACH OF CHILDREN.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults & children 12 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 12 years of age: consult a doctor
Other information
- store at room temperature 68-77°F (20-25°C)
- do not store in direct sunlight or expose to excessive heat and moisture
TAMPER EVIDENT: Do not use if pouch containing the patch is torn or broken.
Inactive ingredients
hydrogenated poly (C6-20 olefin), mineral oil, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, styrene/isoprene copolymer
Manufactured for & Distributed by:
Manufactured for:
Farla International Ltd., Unit 2,
Staples Corner Business Park, 1000 North Circular Road,
London NW2 7JP
Tel +1 (844) 280-8400
sales@farlainternational.com - www.farlainternational.com
Distributed by:
Platinum Care
240 52nd Street, Brooklyn, NY 11220
Tel: +1-718-435-2072
- Pricipal Display Panel
-
INGREDIENTS AND APPEARANCE
MEDIRELIEF HOT
capsaicin patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73486-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.25 mg Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PENTAERYTHRITOL 3,5-DI-TERT-BUTYL-4-HYDROXYHYDROCINNAMATE (UNII: A8VXR0397K) STYRENE/ISOPRENE/STYRENE BLOCK COPOLYMER (UNII: K7S96QM8DV) HYDROGENATED C6-20 POLYOLEFIN (100 CST) (UNII: 39EYQ1W9RB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73486-100-35 5 in 1 BOX 05/31/2020 1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/31/2020 Labeler - Farla Medical Healthcare LTD (230061759)