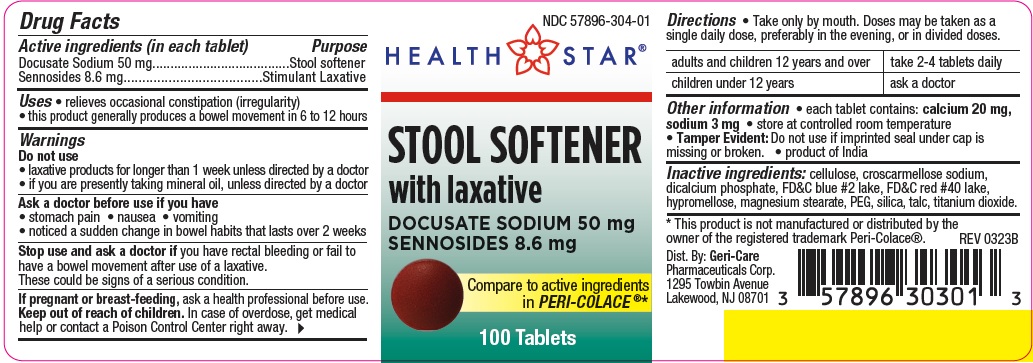
Label: STOOL SOFTENER WITH LAXATIVE- docusate sodium and sennosides tablet, film coated
- NDC Code(s): 57896-304-01, 57896-304-10
- Packager: Geri-Care Pharmaceutical Corp
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 6, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
- laxative products for longer than 1 week unless directed by a doctor
- if you are presently taking mineral oil, unless directed by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Stop use and ask a doctor if you have rectal bleeding or fail to have a bowel movement after use of a laxative. These could
be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
- Directions
- Other information
- Inactive ingredients
- Package Label
-
INGREDIENTS AND APPEARANCE
STOOL SOFTENER WITH LAXATIVE
docusate sodium and sennosides tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57896-304 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) HYPROMELLOSES (UNII: 3NXW29V3WO) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) FD&C RED NO. 40 (UNII: WZB9127XOA) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color red Score no score Shape ROUND Size 10mm Flavor Imprint Code PSD21 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57896-304-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/01/2019 2 NDC:57896-304-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 12/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/01/2019 Labeler - Geri-Care Pharmaceutical Corp (611196254) Registrant - Geri-Care Pharmaceutical Corp (611196254)