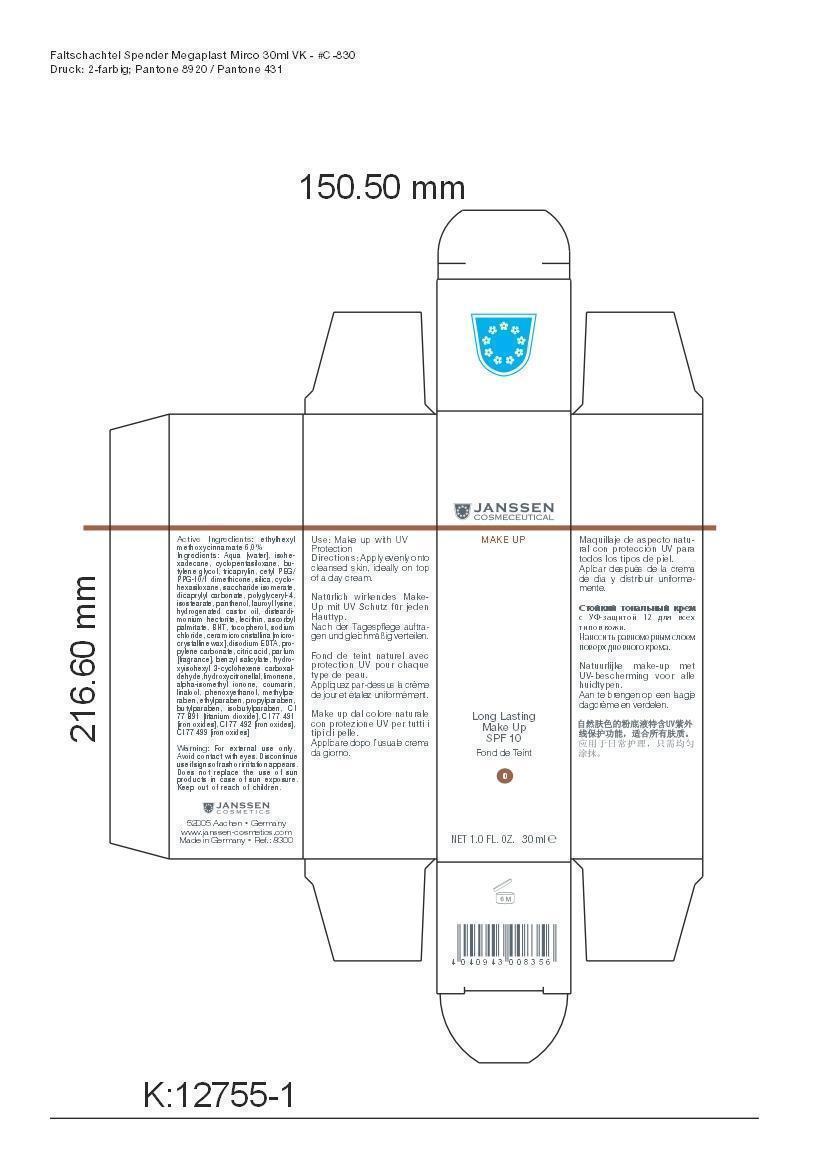
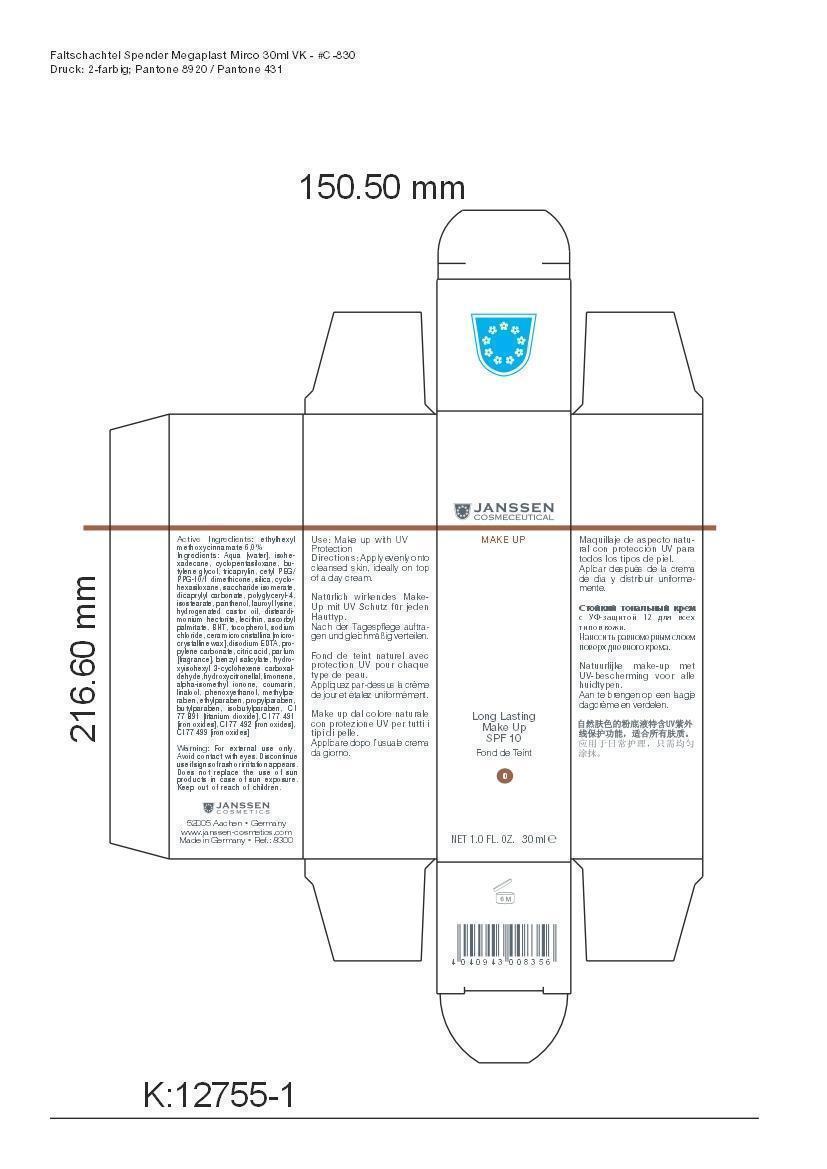
Label: LONG LAST MAKEUP 00- octinoxate cream
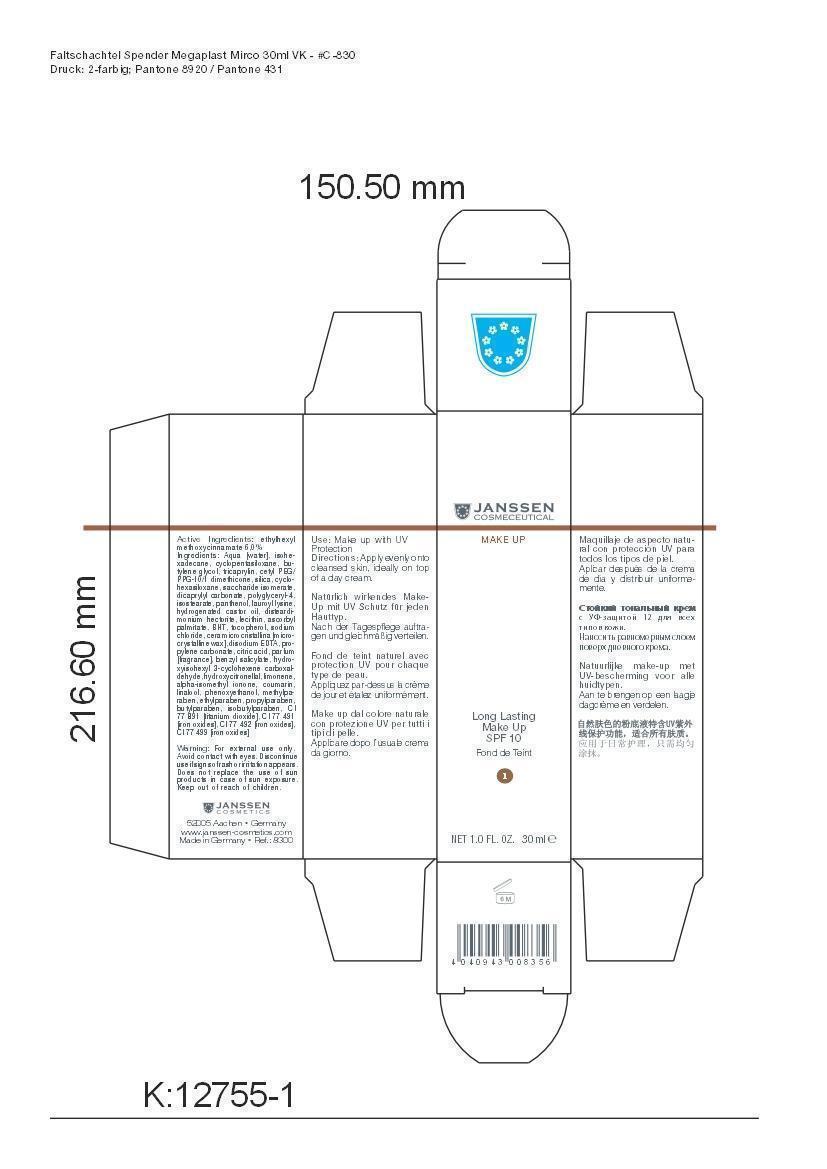
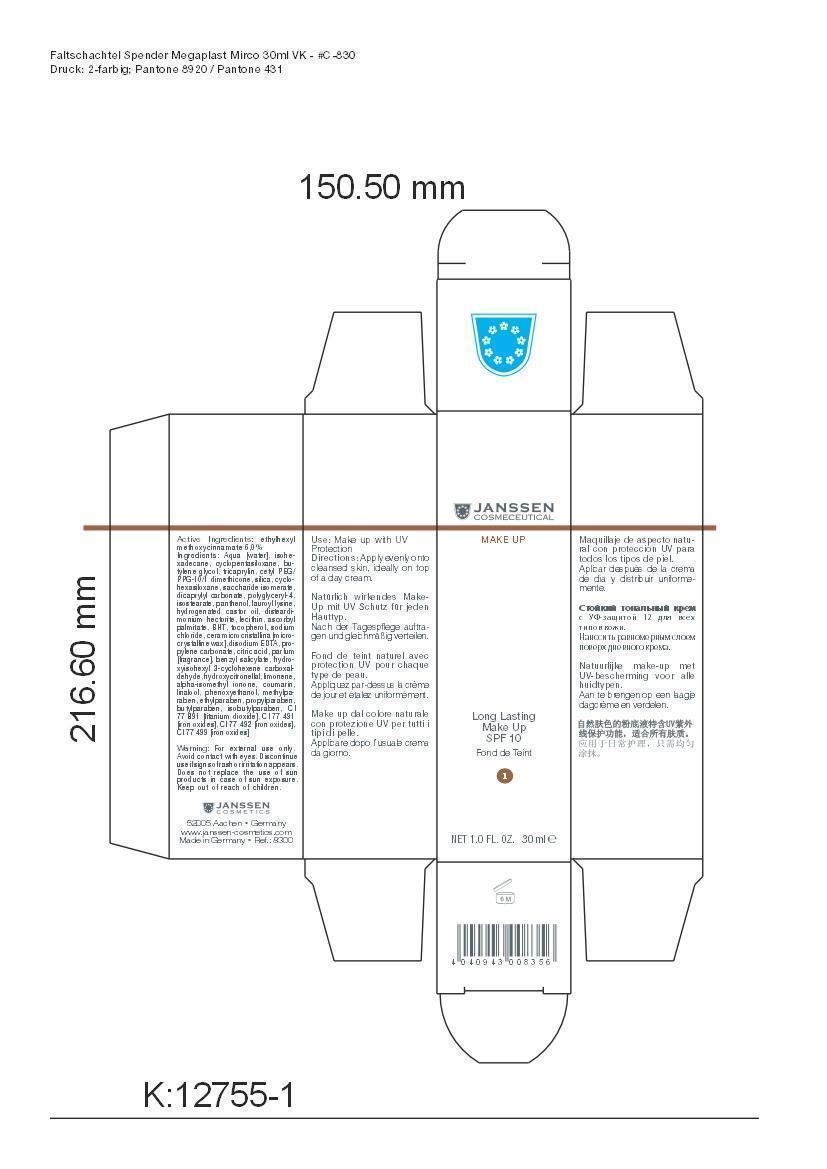
LONG LAST MAKEUP 01- octinoxate cream
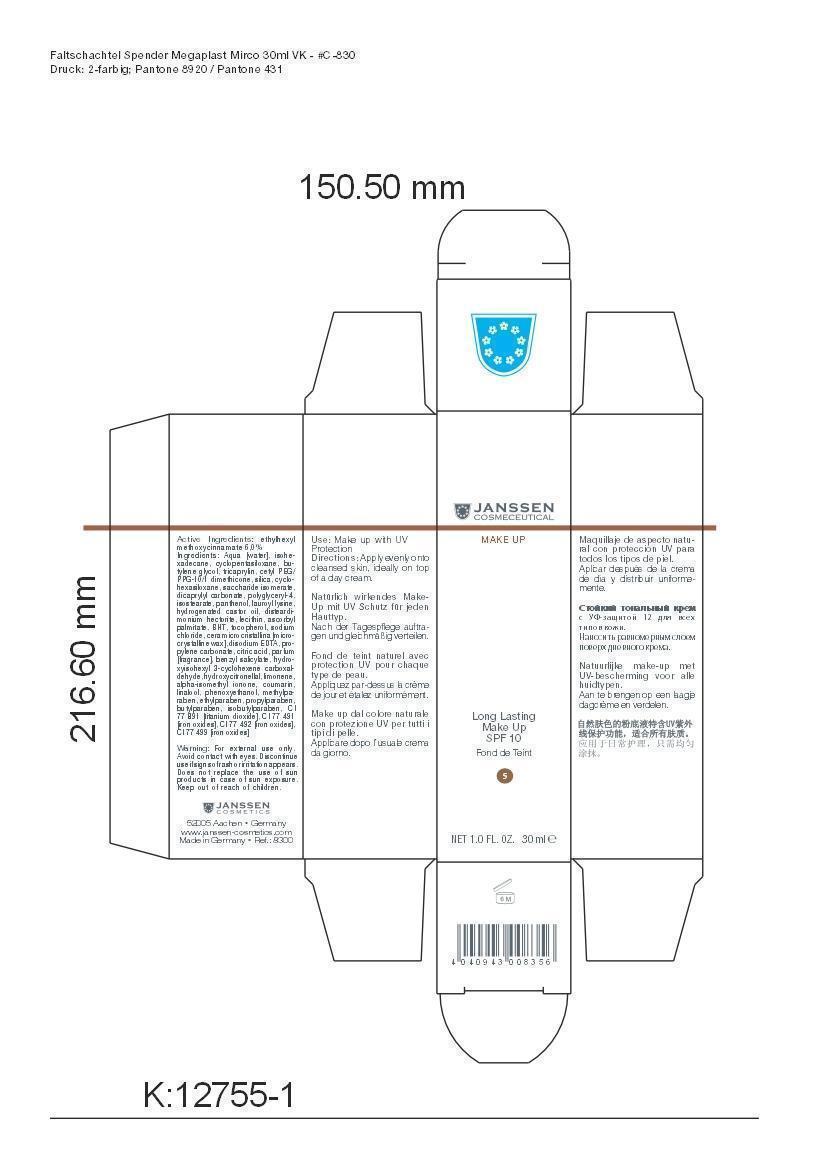
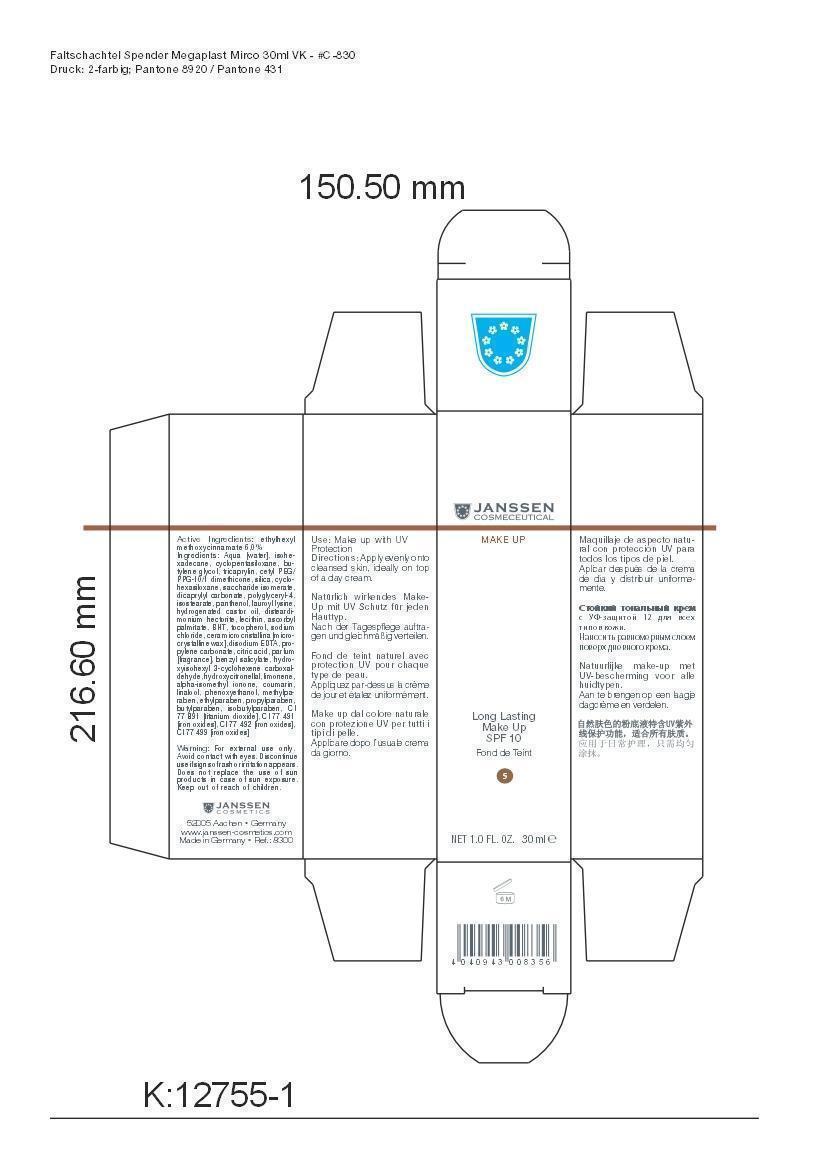
LONG LAST MAKEUP 05- octinoxate cream
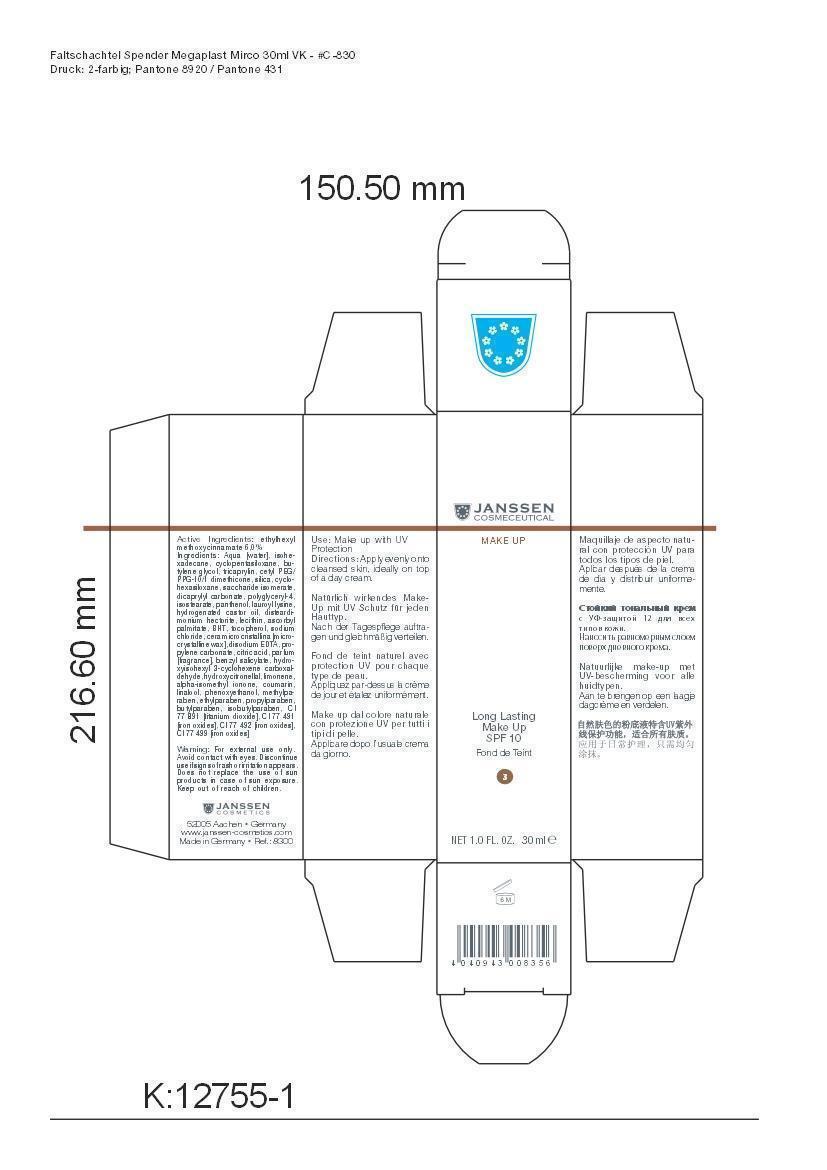
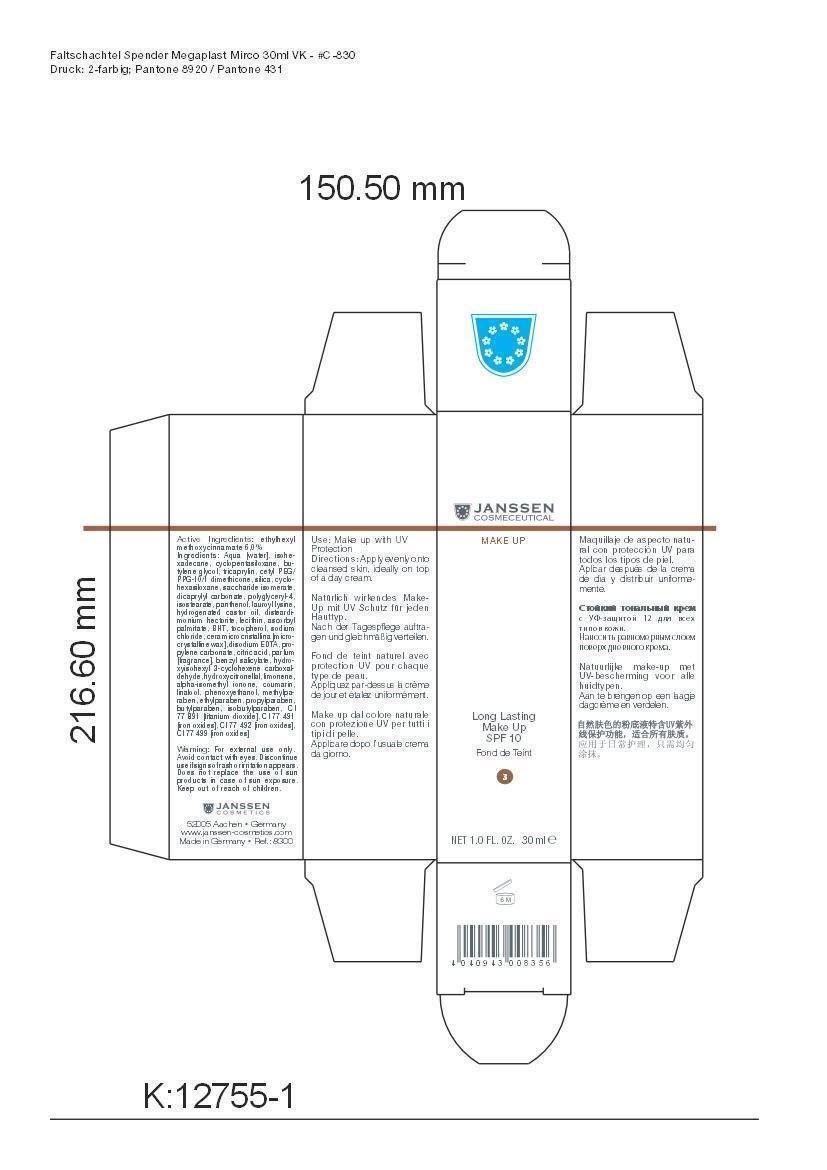
LONG LAST MAKEUP 03- octinoxate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 24653-800-01, 24653-800-02, 24653-801-01, 24653-801-02, view more24653-802-01, 24653-802-02, 24653-803-01, 24653-803-02 - Packager: Janssen Cosmetics GmbH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 11, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Drug Facts:
Active Ingredient: Ethylhexyl methoxycinnamate . . . . . . . . . . 6% Purpose: Sunscreen
Inactive Ingredient
Aqua [water], isohexadecane, cyclopentasiloxane, butylene glycol, tricaprylin, cetyl PEG/PPG-10/1 dimethicone, silica, cyclohexasiloxane, saccharide isomerate, dicaprylyl carbonate, polyglyceryl-4,isostearate, panthenol, lauroyl lysine, hydrogenated castor oil, disteardimonium hectorite, lecithin, ascorbyl palmitate, BHT, tocopherol, sodium chloride, cera microcristallina [microcrystalline wax], disodium EDTA, propylene carbonate, citric acid, parfum [fragrance], benzyl salicylate, hydroxyisohexyl 3-cyclohexene carboxaldehyde, hydroxycitronellal, limonene, alpha-isomethyl ionone, coumarin, linalool, phenoxyethanol, methylparaben, ethylparaben, propylparaben, butylparaben, isobutylparaben, CI 77 891 [titanium dioxide], CI 77 491 [iron oxides], CI 77 492 [iron oxides], CI 77 499 [iron oxides]
- Ask Doctor Section
- Purpose of Use
- Keep out of reach
- Long Last Make UP 00 30ml bottle - 8300
- Long Lasting Makeup 01 30ml pump bottle -8301
- Long Lasting Makeup 03 -30ml pump bottle -8303
- Long Lasting Makeup 05 - 30ml pump bottle - 8305
-
INGREDIENTS AND APPEARANCE
LONG LAST MAKEUP 00
octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24653-800 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.8 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOHEXADECANE (UNII: 918X1OUF1E) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TRICAPRYLIN (UNII: 6P92858988) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 1.5) (UNII: V2W71V8T0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CYCLOMETHICONE 6 (UNII: XHK3U310BA) SACCHARIDE ISOMERATE (UNII: W8K377W98I) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) PANTHENOL (UNII: WV9CM0O67Z) LAUROYL LYSINE (UNII: 113171Q70B) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ASCORBYL PALMITATE (UNII: QN83US2B0N) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM CHLORIDE (UNII: 451W47IQ8X) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) EDETATE DISODIUM (UNII: 7FLD91C86K) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) BENZYL SALICYLATE (UNII: WAO5MNK9TU) HYDROXYISOHEXYL 3-CYCLOHEXENE CARBOXALDEHYDE (UNII: QUE43B9Z2Q) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) LIMONENE, (+)- (UNII: GFD7C86Q1W) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) COUMARIN (UNII: A4VZ22K1WT) LINALOOL, (+)- (UNII: F4VNO44C09) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) PROPYLPARABEN (UNII: Z8IX2SC1OH) BUTYLPARABEN (UNII: 3QPI1U3FV8) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24653-800-02 1 in 1 CARTON 1 NDC:24653-800-01 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 10/05/2012 LONG LAST MAKEUP 01
octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24653-801 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.8 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOHEXADECANE (UNII: 918X1OUF1E) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TRICAPRYLIN (UNII: 6P92858988) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 1.5) (UNII: V2W71V8T0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CYCLOMETHICONE 6 (UNII: XHK3U310BA) SACCHARIDE ISOMERATE (UNII: W8K377W98I) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) PANTHENOL (UNII: WV9CM0O67Z) LAUROYL LYSINE (UNII: 113171Q70B) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ASCORBYL PALMITATE (UNII: QN83US2B0N) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM CHLORIDE (UNII: 451W47IQ8X) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) EDETATE DISODIUM (UNII: 7FLD91C86K) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) BENZYL SALICYLATE (UNII: WAO5MNK9TU) HYDROXYISOHEXYL 3-CYCLOHEXENE CARBOXALDEHYDE (UNII: QUE43B9Z2Q) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) LIMONENE, (+)- (UNII: GFD7C86Q1W) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) COUMARIN (UNII: A4VZ22K1WT) LINALOOL, (+)- (UNII: F4VNO44C09) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) PROPYLPARABEN (UNII: Z8IX2SC1OH) BUTYLPARABEN (UNII: 3QPI1U3FV8) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24653-801-02 1 in 1 CARTON 1 NDC:24653-801-01 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 10/10/2008 LONG LAST MAKEUP 05
octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24653-802 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.8 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOHEXADECANE (UNII: 918X1OUF1E) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TRICAPRYLIN (UNII: 6P92858988) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 1.5) (UNII: V2W71V8T0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CYCLOMETHICONE 6 (UNII: XHK3U310BA) SACCHARIDE ISOMERATE (UNII: W8K377W98I) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) PANTHENOL (UNII: WV9CM0O67Z) LAUROYL LYSINE (UNII: 113171Q70B) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ASCORBYL PALMITATE (UNII: QN83US2B0N) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM CHLORIDE (UNII: 451W47IQ8X) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) EDETATE DISODIUM (UNII: 7FLD91C86K) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) BENZYL SALICYLATE (UNII: WAO5MNK9TU) HYDROXYISOHEXYL 3-CYCLOHEXENE CARBOXALDEHYDE (UNII: QUE43B9Z2Q) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) LIMONENE, (+)- (UNII: GFD7C86Q1W) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) COUMARIN (UNII: A4VZ22K1WT) LINALOOL, (+)- (UNII: F4VNO44C09) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) PROPYLPARABEN (UNII: Z8IX2SC1OH) BUTYLPARABEN (UNII: 3QPI1U3FV8) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24653-802-02 1 in 1 CARTON 1 NDC:24653-802-01 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/10/2012 LONG LAST MAKEUP 03
octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24653-803 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.8 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOHEXADECANE (UNII: 918X1OUF1E) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TRICAPRYLIN (UNII: 6P92858988) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 1.5) (UNII: V2W71V8T0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CYCLOMETHICONE 6 (UNII: XHK3U310BA) SACCHARIDE ISOMERATE (UNII: W8K377W98I) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) PANTHENOL (UNII: WV9CM0O67Z) LAUROYL LYSINE (UNII: 113171Q70B) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ASCORBYL PALMITATE (UNII: QN83US2B0N) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM CHLORIDE (UNII: 451W47IQ8X) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) EDETATE DISODIUM (UNII: 7FLD91C86K) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) BENZYL SALICYLATE (UNII: WAO5MNK9TU) HYDROXYISOHEXYL 3-CYCLOHEXENE CARBOXALDEHYDE (UNII: QUE43B9Z2Q) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) LIMONENE, (+)- (UNII: GFD7C86Q1W) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) COUMARIN (UNII: A4VZ22K1WT) LINALOOL, (+)- (UNII: F4VNO44C09) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) PROPYLPARABEN (UNII: Z8IX2SC1OH) BUTYLPARABEN (UNII: 3QPI1U3FV8) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24653-803-02 1 in 1 CARTON 1 NDC:24653-803-01 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 10/10/2008 Labeler - Janssen Cosmetics GmbH (499187946) Registrant - Janssen Cosmetics GmbH (499187946) Establishment Name Address ID/FEI Business Operations Janssen Cosmecetics GmbH 499187946 manufacture(24653-800) Establishment Name Address ID/FEI Business Operations Dr. Sacher Kosmetik GmbH 341926681 api manufacture(24653-800)