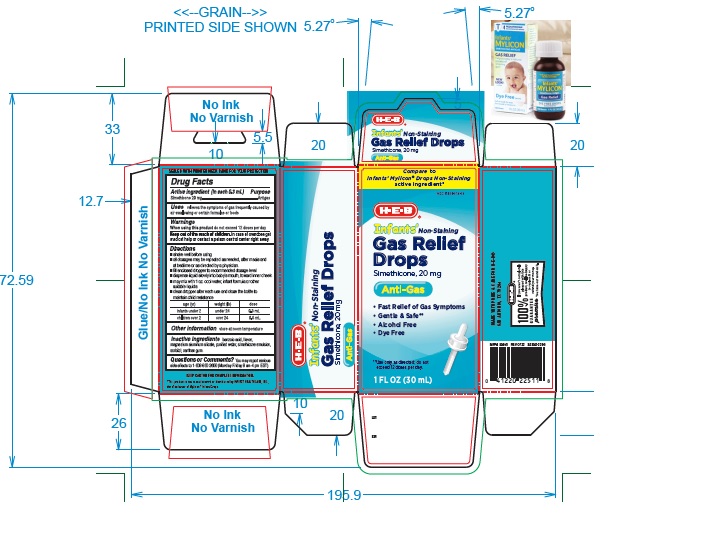
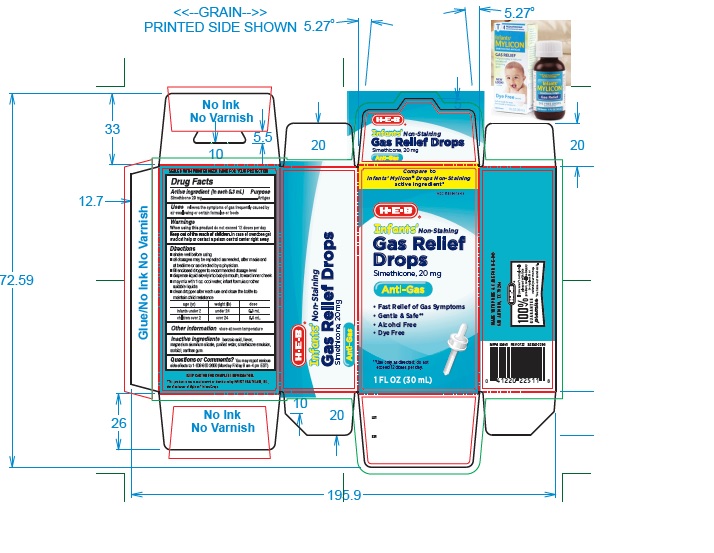
Label: INFANTS NON-STAINING GAS RELIEF- simethicone solution/ drops
- NDC Code(s): 37808-014-09
- Packager: H E B
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT (in each 0.3 mL)
- PURPOSE
- USE(S)
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
- shake well before using
- all dosages may be repeated as needed, after meals and at bedtime or as directed by a physician.
- fill enclosed dropper to recommended dosage level
- dispense liquid slowly into baby's mouth, toward the inner cheek
- may mix with 1 oz. of cool water, infant formula or other suitable liquids.
- clean dropper after each use and close the bottle to maintain child resistance
age (yr) weight (lb) dose infants under 2 under 24 0.3 mL children over 2 over 24 0.6 mL
- OTHER INFORMATION
- INACTIVE INGREDIENT SECTION
- QUESTIONS?
-
PRINCIPAL DISPLAY PANEL
Compare to Infants' Mylicon® Drops Non-Staining active ingredient*
NDC 37808-014-09
HEB
Infants' Non-Staining
Gas Relief
Drops
simethicone 20 mg
Anti-Gas
- Fast Relief of Gas Symptoms
- No Artificial flavor
- Gentle & Safe**
- Alcohol Free
- Dye free
**Use only as directed, do not exceed more than 12 doses per day.
1 FL OZ (30 mL)
-
INGREDIENTS AND APPEARANCE
INFANTS NON-STAINING GAS RELIEF
simethicone solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37808-014 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 20 mg in 0.3 mL Inactive Ingredients Ingredient Name Strength BENZOIC ACID (UNII: 8SKN0B0MIM) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color WHITE (off-white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37808-014-09 1 in 1 CARTON 11/15/2021 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug part332 11/15/2021 Labeler - H E B (007924756) Establishment Name Address ID/FEI Business Operations Guardian Drug Company 119210276 MANUFACTURE(37808-014)