Label: MEDISTIK PROFESSIONAL DUAL ACTION- menthol, methyl salicylate, eucaluptus stick
- NDC Code(s): 50231-221-11
- Packager: Natureteq Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
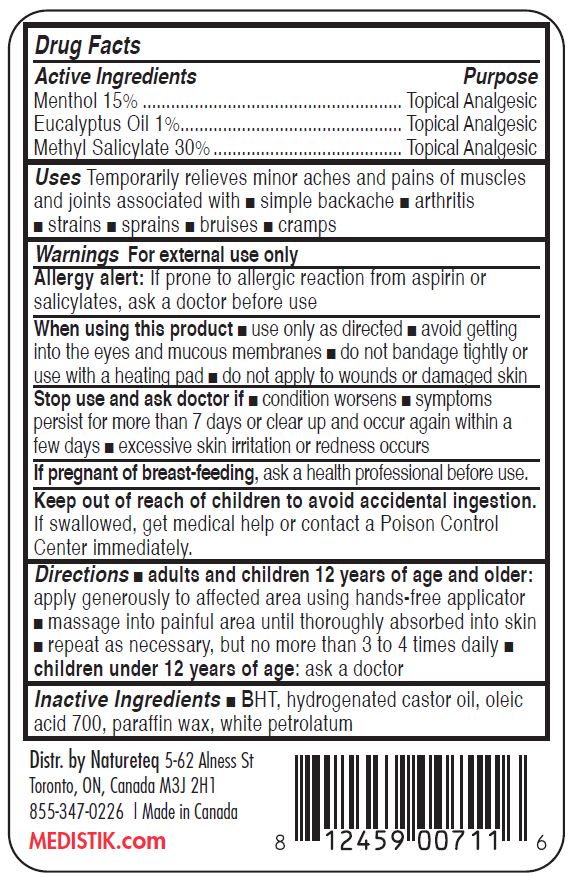
- ACTIVE INGREDIENTS
- PURPOSE
- USES
-
WARNINGS
For external use only. The application of external heat may cause skin irritation or burn. Avoid contact with eyes and broken skin. Do not bandage. DO NOT USE IF ALLERGIC TO SALICYLATES OR IF TAKING ANTICOAGULANT MEDICATIONS. Stop use and consult a physician if condition worsens, rash or irritation develops, or if symptoms persist for more than 7 days or clear up and recur in a few days.
-
DIRECTIONS
For use on adults and children over 12 years of age. Apply generously to the affected area using hands-free applicator. Use 3 to 4 times per day as required. A stinging or burning sensation will be experienced during the first few minutes as the formula begins working. For arthritis or muscle pain of the hands, retain for at least 10 minutes then wash hands.
- INACTIVE INGREDIENTS
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MEDISTIK PROFESSIONAL DUAL ACTION
menthol, methyl salicylate, eucaluptus stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50231-221 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 15 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 30 g in 100 g EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) (EUCALYPTUS GLOBULUS LEAF - UNII:S546YLW6E6) EUCALYPTUS GLOBULUS LEAF 1 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) OLEIC ACID (UNII: 2UMI9U37CP) PARAFFIN (UNII: I9O0E3H2ZE) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50231-221-11 58 g in 1 CANISTER; Type 0: Not a Combination Product 04/15/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 04/15/2011 Labeler - Natureteq Inc. (243737371)