Label: PREVACID 24 HR- lansoprazole capsule, delayed release
- NDC Code(s): 0113-6002-01, 0113-6002-03
- Packager: L. Perrigo Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
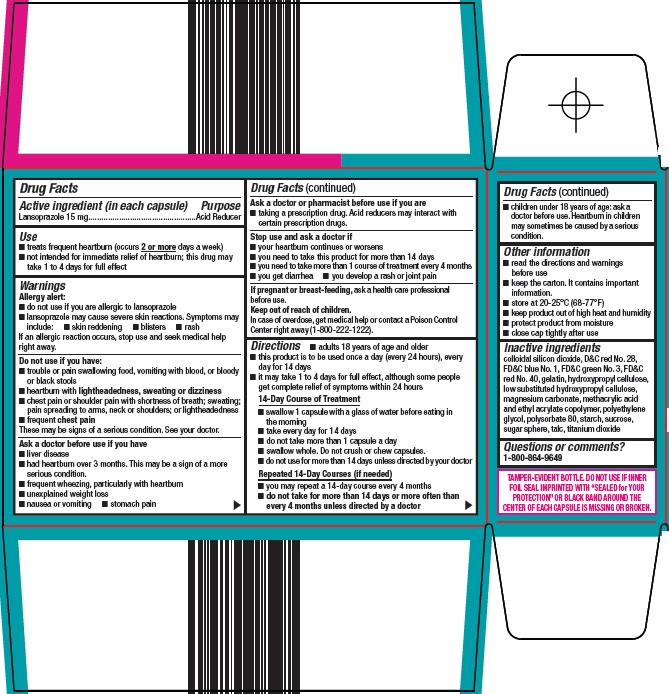
- Active ingredient (in each capsule)
- Purpose
- Use
-
Warnings
Allergy alert:
- •
- do not use if you are allergic to lansoprazole
- •
- lansoprazole may cause severe skin reactions. Symptoms may include:
- •
- skin reddening
- •
- blisters
- •
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
Do not use if you have:
- •
- trouble or pain swallowing food, vomiting with blood, or bloody or black stools
- •
- heartburn with lightheadedness, sweating or dizziness
- •
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- •
- frequent chest pain
- These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- •
- liver disease
- •
- had heartburn over 3 months. This may be a sign of a more serious condition.
- •
- frequent wheezing, particularly with heartburn
- •
- unexplained weight loss
- •
- nausea or vomiting
- •
- stomach pain
Ask a doctor or pharmacist before use if you are
- •
- taking a prescription drug. Acid reducers may interact with certain prescription drugs.
-
Directions
- •
- adults 18 years of age and older
- •
- this product is to be used once a day (every 24 hours), every day for 14 days
- •
- it may take 1 to 4 days for full effect, although some people get complete relief of symptoms within 24 hours
14-Day Course of Treatment
- •
- swallow 1 capsule with a glass of water before eating in the morning
- •
- take every day for 14 days
- •
- do not take more than 1 capsule a day
- •
- swallow whole. Do not crush or chew capsules.
- •
- do not use for more than 14 days unless directed by your doctor
Repeated 14-Day Courses (if needed)
- •
- you may repeat a 14-day course every 4 months
- •
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- •
- children under 18 years of age: ask a doctor before use. Heartburn in children may sometimes be caused by a serious condition.
- Other information
-
Inactive ingredients
colloidal silicon dioxide, D&C red No. 28, FD&C blue No. 1, FD&C green No. 3, FD&C red No. 40, gelatin, hydroxypropyl cellulose, low substituted hydroxypropyl cellulose, magnesium carbonate, methacrylic acid and ethyl acrylate copolymer, polyethylene glycol, polysorbate 80, starch, sucrose, sugar sphere, talc, titanium dioxide
- Questions or comments?
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PREVACID 24 HR
lansoprazole capsule, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0113-6002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANSOPRAZOLE (UNII: 0K5C5T2QPG) (LANSOPRAZOLE - UNII:0K5C5T2QPG) LANSOPRAZOLE 15 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) MAGNESIUM CARBONATE (UNII: 0E53J927NA) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BLUE (TEAL) , PINK Score no score Shape CAPSULE Size 16mm Flavor Imprint Code P24HR Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0113-6002-01 1 in 1 CARTON 11/05/2020 1 14 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:0113-6002-03 3 in 1 CARTON 11/19/2020 2 14 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022327 11/05/2020 Labeler - L. Perrigo Company (006013346)