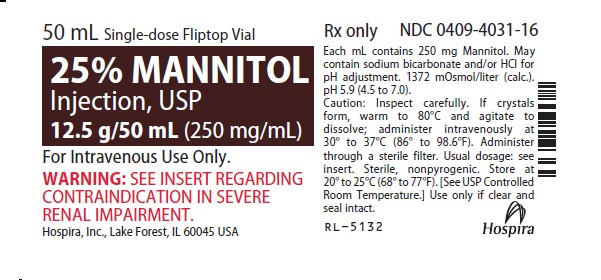
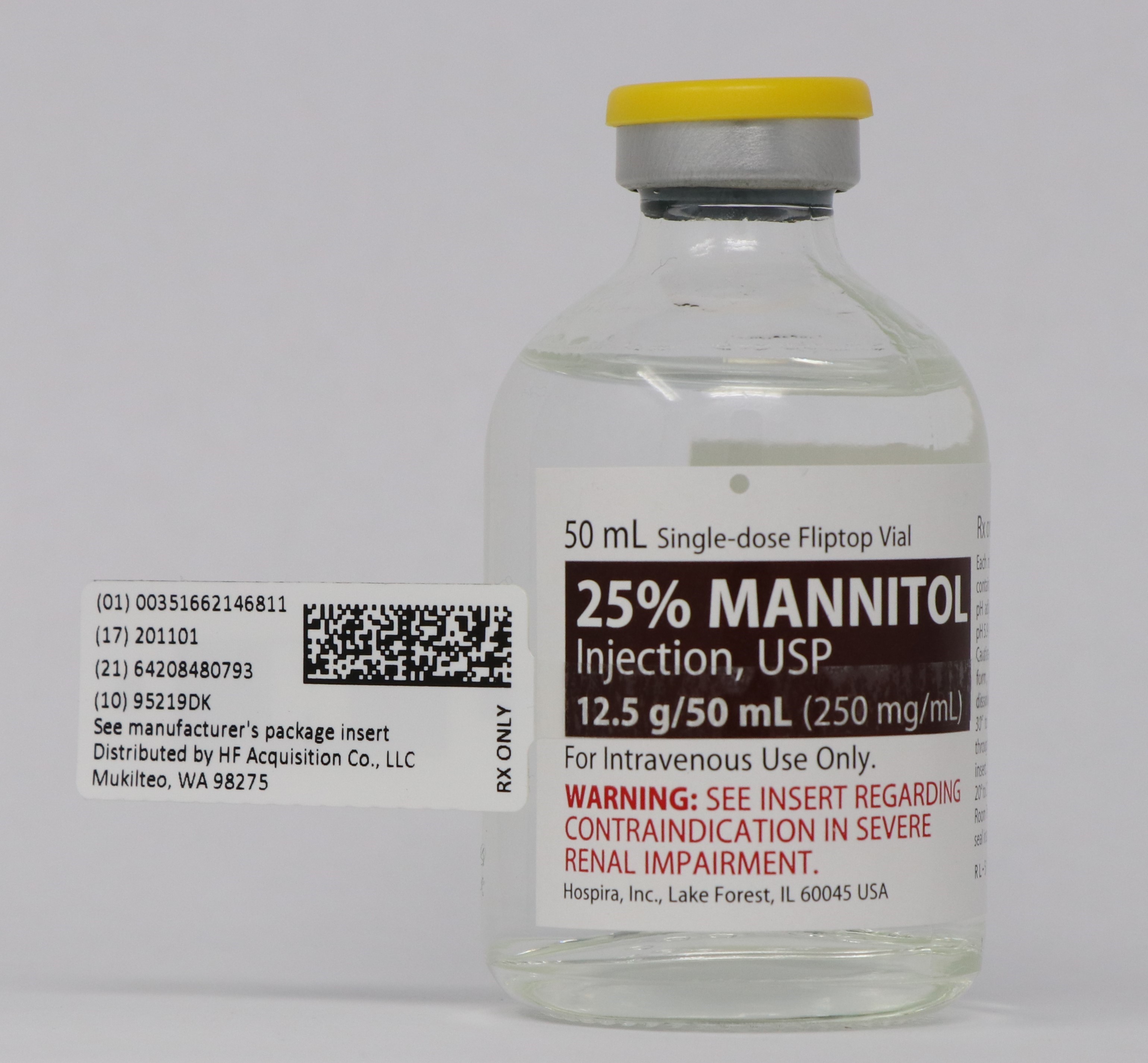
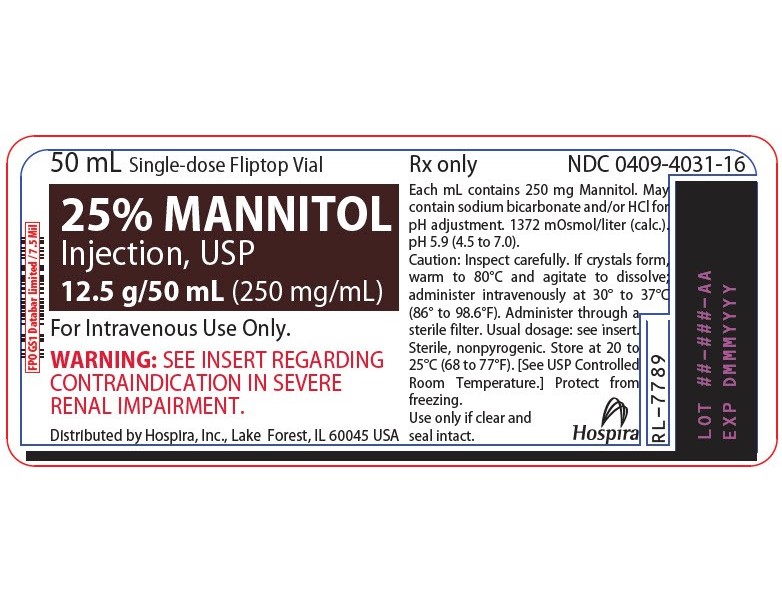
Label: MANNITOL injection, solution
- NDC Code(s): 51662-1468-1, 51662-1468-2, 51662-1468-3
- Packager: HF Acquisition Co LLC, DBA HealthFirst
- This is a repackaged label.
- Source NDC Code(s): 0409-4031
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MANNITOL INJECTION safely and effectively. See full prescribing information for MANNITOL INJECTION.
MANNITOL injection, for intravenous use
Initial U.S. Approval: 1964INDICATIONS AND USAGE
Mannitol Injection is indicated for the following purposes in adults and pediatric patients.
Therapeutic Use
Reduction of intracranial pressure and brain mass. ( 1)
Reduction of high intraocular pressure. ( 1)Diagnostic Use
Measurement of glomerular filtration rate. ( 1)
DOSAGE AND ADMINISTRATION
Administration Instructions ( 2-2.1):
For intravenous use only.
Do not add mannitol in whole blood for transfusion.Recommended Dosage ( 2-2.2):
The dosage, concentration and rate of administration depend on the age, weight and condition of the patient.
Reduction of Intracranial Pressure and Brain Mass:
Adults: 0.25 to 2 g/kg body weight as a 15% to 25% solution administered over a period of 30 to 60 minutes
Pediatric patients: 1 to 2 g/kg body weight or 30 to 60 g/m2 body surface area over a period of 30 to 60 minutes.
Small or debilitated patients: 500 mg/kg
Reduction of Intraocular Pressure:
Adults: 0.25 to 2 g/kg body weight as a 15% to 25% solution administered over a period of 30 to 60 minutes
Pediatric patients: 1 to 2 g/kg body weight or 30 to 60 g/m2 body surface area over a period of 30 to 60 minutes
Small or debilitated patients: 500 mg/kg
Measurement of Glomerular Filtration Rate (GFR):
100 mL of a 20% solution (20 g) should be diluted with 180 mL of sodium chloride injection (normal saline) or 200 mL of a 10% solution (20 g) should be diluted with 80 mL of sodium chloride injection (normal saline). The resulting 280 mL of 7.2% solution is infused at a rate of 20 mL per minute.DOSAGE FORMS AND STRENGTHS
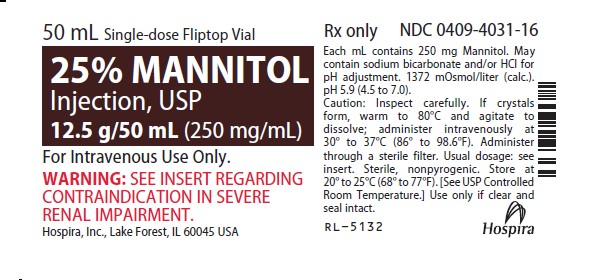
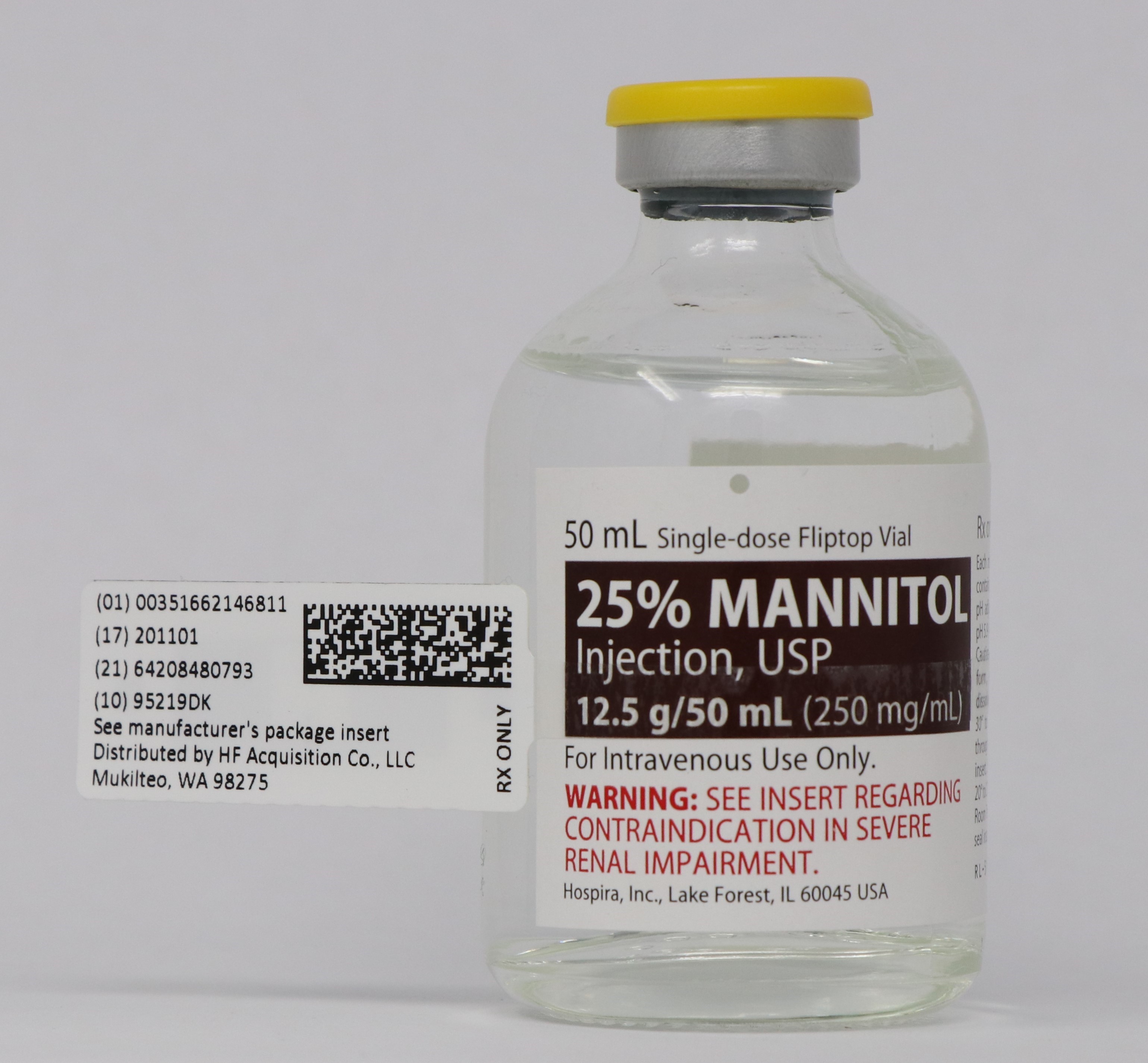
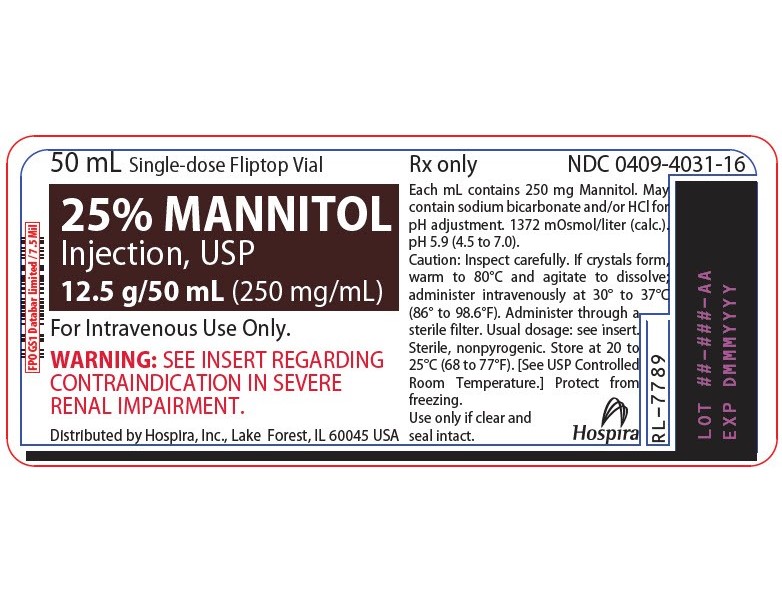
Mannitol Injection, USP: 25% (250 mg/mL): 25 grams of mannitol, USP per 100 mL in a single-dose 50 mL fliptop vial (3)
CONTRAINDICATIONS
Well established anuria due to severe renal disease. ( 4)
Severe pulmonary congestion or frank pulmonary edema. ( 4)
Active intracranial bleeding except during craniotomy. ( 4)
Severe dehydration. ( 4)
Progressive heart failure or pulmonary congestion after institution of mannitol therapy. ( 4)Do not administer to patients with a known hypersensitivity to mannitol. ( 4)
WARNINGS AND PRECAUTIONS
Renal Complications Including Renal Failure: Risk factors include pre-existing renal disease, conditions that put patients at risk for renal failure and concomitant use of nephrotoxic drugs or other diuretics. Avoid concomitant administration of nephrotoxic drugs or other diuretics with mannitol. ( 5-5.1, 8-8.6)
Fluid and Electrolyte Imbalances: Mannitol administration may obscure and intensify inadequate hydration or hypovolemia. Excessive loss of water and electrolytes may lead to serious imbalances, e.g., hypernatremia, hyponatremia. Accumulation of mannitol may intensify existing or latent congestive heart failure. Monitoring of cardiovascular status and electrolyte levels is recommended. ( 5-5.2)
Central Nervous System (CNS) Toxicity: Mannitol may increase cerebral blood flow and the risk of postoperative bleeding in neurosurgical patients. It may also worsen intracranial hypertension in children who develop a generalized cerebral hyperemia during the first 24 to 48 hours post injury. ( 5-5.3)
Monitoring: Discontinue mannitol if renal, cardiac or pulmonary status worsens, or CNS toxicity develops. ( 5-5.4)ADVERSE REACTIONS
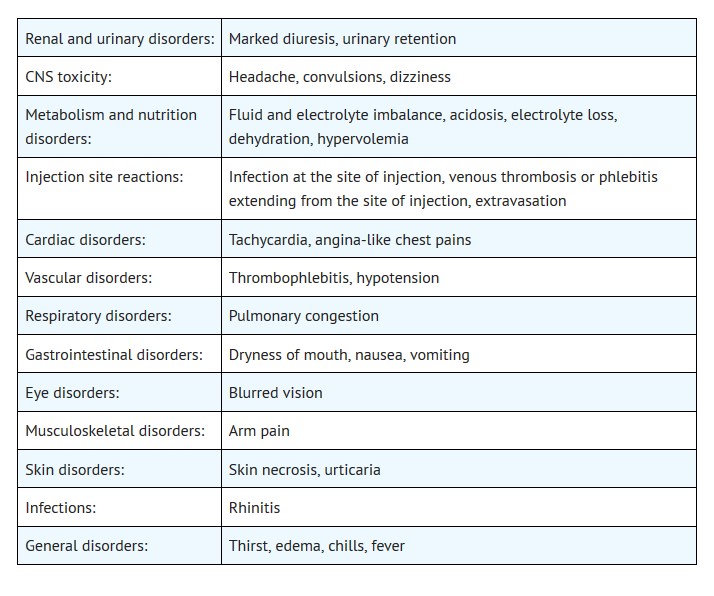
Most common adverse reactions are pulmonary congestion, fluid and electrolyte imbalance, acidosis, electrolyte loss, dryness of mouth, thirst, marked diuresis, urinary retention, edema, headache, blurred vision, convulsions, nausea, vomiting, rhinitis, arm pain, skin necrosis, thrombophlebitis, chills, dizziness, urticaria, dehydration, hypotension, tachycardia, fever and angina-like chest pains. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Hospira, Inc. at 1-800-441-4100 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Nephrotoxic Drugs and Diuretics: May increase the risk of renal failure; avoid concomitant use. ( 7-7.1, 7-7.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2019
-
TABLE OF CONTENTS
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
2.2 Recommended Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Renal Complications Including Renal Failure
5.2 Fluid and Electrolyte Imbalances
5.3 Central Nervous System (CNS) Toxicity
5.4 Monitoring
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Nephrotoxic Drugs
7.2 Diuretics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION*
Sections or subsections omitted from the full prescribing information are not listed. - 1 INDICATIONS & USAGE
-
2 DOSAGE & ADMINISTRATION
2.1 Important Preparation and Administration Instructions
For intravenous use only. Do not administer intramuscularly or subcutaneously. Never add mannitol in whole blood for transfusion.
Do not administer unless solution is clear and container is undamaged. Discard unused portion. Do not administer 25% mannitol if the fliptop vial seal is not intact.
Additives may be incompatible. Consult with pharmacist, if available.
Do not place 25% Mannitol Injection in polyvinylchloride (PVC) bags; a white flocculent precipitate may form from contact with PVC surfaces. Parenteral drug products should be inspected visually for particulate matter and discoloration whenever container and solution permit.
2.2 Recommended Dosage
Reduction of Intracranial Pressure and Brain Mass
In adults a dose of 0.25 to 2 g/kg body weight as a 15% to 25% solution administered over a period of 30 to 60 minutes; pediatric patients 1 to 2 g/kg body weight or 30 to 60 g/m2 body surface area over a period of 30 to 60 minutes. In small or debilitated patients, a dose of 500 mg/kg may be sufficient. Careful evaluation must be made of the circulatory and renal reserve prior to and during administration of mannitol at the higher doses and rapid infusion rates. Careful attention must be paid to fluid and electrolyte balance, body weight, and total input and output before and after infusion of mannitol. Evidence of reduced cerebral spinal fluid pressure must be observed within 15 minutes after starting infusion.
Reduction of Intraocular Pressure
In adults a dose of 0.25 to 2 g/kg body weight as a 15% to 25% solution administered over a period of 30 to 60 minutes; pediatric patients 1 to 2 g/kg body weight or 30 to 60 g/m2 body surface area over a period of 30 to 60 minutes. In small or debilitated patients, a dose of 500 mg/kg may be sufficient. When used preoperatively, the dose should be given one to one and one-half hours before surgery to achieve maximal reduction of intraocular pressure before operation.
Measurement of Glomerular Filtration Rate (GFR)
100 mL of a 20% solution (20 g) should be diluted with 180 mL of sodium chloride injection (normal saline) or 200 mL of a 10% solution (20 g) should be diluted with 80 mL of sodium chloride injection (normal saline). The resulting 280 mL of 7.2% solution is infused at a rate of 20 mL per minute. The urine is collected by catheter for a specific period of time and analyzed for mannitol excreted in mg per minute. A blood sample is drawn at the start and at the end of the time period and the concentration of mannitol determined in mg/mL of plasma. GFR is the number of mL of plasma that must have been filtered to account for the amount excreted per minute in the urine. Normal clearance rates are approximately 125 mL/minute for men; 116 mL/minute for women.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Mannitol Injection is contraindicated in patients with:
Well established anuria due to severe renal disease.
Severe pulmonary congestion or frank pulmonary edema.
Active intracranial bleeding except during craniotomy.
Severe dehydration.
Progressive heart failure or pulmonary congestion after institution of mannitol therapy.Do not administer to patients with a known hypersensitivity to mannitol.
-
5 WARNINGS AND PRECAUTIONS
5.1 Renal Complications Including Renal Failure
Renal complications, including irreversible renal failure have been reported in patients receiving mannitol.
Reversible, oliguric acute kidney injury (AKI) has occurred in patients with normal pretreatment renal function who received mannitol.
Osmotic nephrosis, a reversible vacuolization of the tubules of no known clinical significance, may proceed to severe irreversible nephrosis, so that the renal function must be closely monitored during mannitol infusion.
Patients with pre-existing renal disease, patients with conditions that put them at risk for renal failure, or those receiving potentially nephrotoxic drugs or other diuretics, are at increased risk for renal failure. Avoid concomitant administration of nephrotoxic drugs (e.g., aminoglycosides) or other diuretics with mannitol [see Drug Interactions (7)].
If urine output declines during mannitol infusion, the patient's clinical status should be closely reviewed and mannitol infusion suspended if necessary.
5.2 Fluid and Electrolyte Imbalances
The obligatory diuretic response following rapid infusion of 25% mannitol may further aggravate pre-existing hemoconcentration. Excessive loss of water and electrolytes may lead to serious imbalances. With continued administration of mannitol, loss of water in excess of electrolytes can cause hypernatremia. Electrolyte measurements, including sodium and potassium are therefore of vital importance in monitoring the infusion of mannitol.
The shift of sodium-free intracellular fluid into the extracellular compartment following mannitol infusion may lower serum sodium concentration and aggravate pre-existing hyponatremia.
By sustaining diuresis, mannitol administration may obscure and intensify inadequate hydration or hypovolemia.
Accumulation of mannitol may result in overexpansion of the extracellular fluid which may intensify existing or latent congestive heart failure.
The cardiovascular status of the patient should be carefully evaluated before rapidly administering mannitol since sudden expansion of the extracellular fluid may lead to fulminating congestive heart failure.
5.3 Central Nervous System (CNS) Toxicity
Mannitol injection may increase cerebral blood flow and the risk of postoperative bleeding in neurosurgical patients.
Mannitol may increase cerebral blood flow and worsen intracranial hypertension in children who develop generalized cerebral hyperemia during the first 24 to 48 hours post injury.
5.4 Monitoring
Serum sodium and potassium should be carefully monitored during mannitol administration.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic counter measures and save the remainder of the fluid for examination if deemed necessary.
Electrolyte-free mannitol solutions should not be given conjointly with blood. If it is essential that blood be given simultaneously, at least 20 mEq of sodium chloride should be added to each liter of mannitol solution to avoid pseudoagglutination.
When exposed to low temperatures, solutions of mannitol may crystallize. If crystals are observed, the container should be warmed to redissolve, then cooled to body temperature before administering [see How Supplied/Storage and Handling (16)]. When infusing 25% mannitol, the administration set should include a filter. Do not infuse mannitol solution if crystals are present.
-
6 ADVERSE REACTIONS
The following adverse reactions associated with the use mannitol were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
-
7 DRUG INTERACTIONS
7.1 Nephrotoxic Drugs
Patients receiving potentially nephrotoxic drugs are at increased risk for renal failure. Avoid concomitant administration of nephrotoxic drugs (e.g., aminoglycosides) with mannitol [see Warnings and Precautions (5)].
7.2 Diuretics
Pateints receiving other diuretics are at increased risk for renal failure. Avoid concomitant administration of other diuretics with mannitol [see Warnings and Precautions (5)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no well-controlled studies in pregnant women that establish developmental toxicity related to the use of mannitol injection. Mannitol crosses the placenta and may cause fluid shifts that could potentially result in adverse effects in the fetus (see Data). The available data do not establish the presence or absence of developmental toxicity related to the use of mannitol.
There are limited animal data in the published literature. The effects of mannitol on embryo-fetal development have not been evaluated; however, fluid shifts occurred in animal studies in response to maternal infusion of mannitol.
Mannitol injection should be given to a pregnant woman only if clearly needed.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Data
Human Data
Published literature reports the presence of mannitol in amniotic fluid when mannitol is administered to pregnant women during the third trimester of pregnancy.
8.2 Lactation
Risk Summary
Lactation studies have not been conducted with mannitol. It is not known whether this drug is excreted in human milk. Mannitol injection should be administered to lactating women only if clearly indicated. Studies assessing the effects of mannitol injection in breastfed children have not been performed. Studies to assess the effect of mannitol on milk production or excretion have not been performed.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for mannitol and any potential adverse effects on the breastfed child from mannitol or from the underlying maternal condition.
8.5 Geriatric Use
Mannitol is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in elderly patients with impaired renal function. Evaluate the renal, cardiac and pulmonary status of the patient and correct fluid and electrolyte imbalances prior to administration of mannitol [see Warnings and Precautions (5)].
8.6 Renal Impairment
Patients with pre-existing renal disease, patients with conditions that put them at high risk for renal failure, or those receiving potentially nephrotoxic drugs or other diuretics, are at increased risk of renal failure with administration of mannitol. Evaluate the renal, cardiac and pulmonary status of the patient and correct fluid and electrolyte imbalances prior to administration of mannitol [see Warnings and Precautions (5)].
-
10 OVERDOSAGE
Too rapid infusion of large amounts of mannitol will cause a shift of intracellular water into the extracellular compartment resulting in cellular dehydration and overexpansion of the intravascular space with hyponatremia, congestive heart failure and pulmonary edema. Repeated doses should not be given to patients with persistent oliguria as this can produce a hyperosmolar state and precipitate congestive heart failure and pulmonary edema due to volume overload. Dosage must be carefully monitored and adjusted in accordance with the clinical situation to avoid the consequences of overdosage [see Contraindications (4), Warnings and Precautions (5), Dosage and Administration (2)].
-
11 DESCRIPTION
Mannitol Injection, USP is a sterile, nonpyrogenic solution of mannitol in water for injection available in a concentration of 25% in a fliptop vial for administration by intravenous infusion only.
The content and characteristics are as follows:
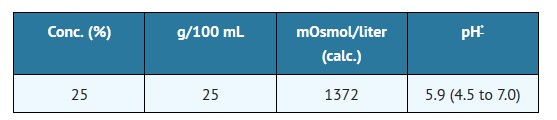
The solution contains no bacteriostat, antimicrobial agent or added buffer (except for pH adjustment) and is intended only as a single-dose injection. When smaller doses are required, the unused portion should be discarded.
Mannitol Injection, USP is a parenteral obligatory osmotic diuretic.
Mannitol, USP is chemically designated D-mannitol (C6H14O6), a white crystalline powder or free-flowing granules freely soluble in water. It has the following structural formula:
*
May contain sodium bicarbonate and/or hydrochloric acid for pH adjustment.25 25 1372 5.9 (4.5 to 7.0)
Water for Injection, USP is chemically designated H2O.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mannitol, when administered intravenously, exerts its osmotic diuretic effect as a solute of relatively small molecular size largely confined to the extracellular space. Mannitol hinders tubular reabsorption of water and enhances excretion of sodium and chloride by elevating the osmolarity of the glomerular filtrate.
By increasing the osmotic pressure of plasma and the extracellular space, intravenously administered mannitol will induce the movement of intracellular water to the extracellular and vascular spaces. This action underlies the role of mannitol in reducing intracranial pressure, intracranial edema, and intraocular pressure.
12.3 Pharmacokinetics
Distribution
Mannitol distributes largely to the extracellular space within 20 to 40 minutes after intravenous administration. The plasma half-life for this distribution phase is 0.16 hour. The volume of distribution of mannitol is approximately 17 L in adults.
Elimination
In subjects with normal renal function, the total clearance is 87 to 109 mL/minute. The elimination half-life of mannitol is 0.5 to 2.5 hours
Metabolism
Only a relatively small amount of the mannitol dose is metabolized after intravenous administration to healthy subjects.
Excretion
Approximately 80% of a 100 g dose appears in the urine in 3 hours. The drug is freely filtered by the glomeruli, with less than 10% tubular reabsorption; it is not secreted by tubular cells.
Specific Populations
Patients with Renal Impairment
In patients with renal impairment, the elimination half-life of mannitol is prolonged. In a published study, in patients with renal impairment including acute renal failure and end stage renal failure, the elimination half-life of mannitol was estimated at about 36 hours, based on serum osmolarity. In patients with renal impairment on dialysis, the elimination half-life of mannitol was reduced to 6 and 21 hours during hemodialysis and peritoneal dialysis, respectively.
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Mannitol Injection, USP is supplied in single-dose containers as follows:
25% MANNITOL INJECTION, USP is supplied in the following dosage forms.
NDC 51662-1468-1
25% MANNITOL INJECTION, USP 12.5g/50mL (250mg/mL) 50mL VIALNDC 51662-1468-2
25% MANNITOL INJECTION, USP 12.5g/50mL (250mg/mL) 50mL VIAL, 1 VIAL PER POUCHNDC 51662-1468-3
25% MANNITOL INJECTION, USP 12.5g/50mL (250mg/mL) 50mL VIAL, 1 VIAL PER POUCH, 25 POUCHES PER CASEHF Acquisition Co LLC, DBA HealthFirst
Mukilteo, WA 98275NOTE: Crystals may form in mannitol solutions especially if the solutions are chilled. To dissolve the crystals, warm the bottle in hot water at 80°C and periodically shake vigorously. 25% Mannitol Injection, USP may be autoclaved at 121°C for 20 minutes at 15 psi. Remove cover from fliptop vial and cleanse stopper with antiseptic before use. Cool to body temperature or less before administering. When infusing 25% mannitol concentrations, the administration set should include a filter.
Protect from freezing. Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
INSTRUCTlONS FOR USE
Remove cover and cleanse stopper with antiseptic before use.
-
17 PATIENT COUNSELING INFORMATION
Inform patients or caregivers of the following risks of Mannitol Injection:
Renal Complications Including Renal Failure [see Warnings and Precautions (5.1)].
Fluid and Electrolyte Imbalances [see Warnings and Precautions (5.2)]
CNS Toxicity [see Warnings and Precautions (5.3)] - SPL UNCLASSIFIED
- PRINCIPAL DISPLAY PANEL - VIAL LABEL OPT. 1
- PRINCIPAL DISPLAY PANEL - VIAL LABEL OPT. 2
- PRINCIPAL DISPLAY PANEL - VIAL LABEL OPT. 3
- PRINCIPAL DISPLAY PANEL - VIAL LABEL OPT. 4
- PRINCIPAL DISPLAY PANEL - SERIALIZED VIAL LABELING
- PRINCIPAL DISPLAY PANEL - NDC 51662-1468-2 POUCH LABELING
- PRINCIPAL DISPLAY PANEL - NDC 51662-1468-3 CASE LABELING
-
INGREDIENTS AND APPEARANCE
MANNITOL
mannitol injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51662-1468(NDC:0409-4031) Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MANNITOL (UNII: 3OWL53L36A) (MANNITOL - UNII:3OWL53L36A) MANNITOL 12.5 g in 50 mL Inactive Ingredients Ingredient Name Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51662-1468-1 50 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 12/13/2019 2 NDC:51662-1468-3 25 in 1 CASE 12/11/2022 2 NDC:51662-1468-2 1 in 1 POUCH 2 50 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA016269 12/13/2019 Labeler - HF Acquisition Co LLC, DBA HealthFirst (045657305) Registrant - HF Acquisition Co LLC, DBA HealthFirst (045657305) Establishment Name Address ID/FEI Business Operations HF Acquisition Co LLC, DBA HealthFirst 045657305 relabel(51662-1468)