Label: HOT/COLD MEDICATED PATCH- hot cold patch patch
- NDC Code(s): 67777-201-10, 67777-201-11, 67777-201-20, 67777-201-21
- Packager: Dynarex
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 27, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
-
Warnings
For external use only
Do not use
- On wounds or damaged skin
- With a heating pad
- If you are allergic to any ingredients of this product
When using this product
- Use only as directed
- Avoid contact with the eyes, mucous membranes or rashes
- Directions
- Other information
- Inactive ingredients
- Questions and Comments?
- Label
- Label
-
INGREDIENTS AND APPEARANCE
HOT/COLD MEDICATED PATCH
hot cold patch patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67777-201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 285 mg in 5700 mg Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CASTOR OIL (UNII: D5340Y2I9G) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) KAOLIN (UNII: 24H4NWX5CO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67777-201-10 180 in 1 CASE 12/13/2019 1 NDC:67777-201-11 5 in 1 BOX 1 285 mg in 1 PATCH; Type 0: Not a Combination Product 2 NDC:67777-201-20 180 in 1 CASE 12/13/2019 2 NDC:67777-201-21 5 in 1 BOX 2 285 mg in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/13/2019 Labeler - Dynarex (008124539) Registrant - Dynarex (008124539)

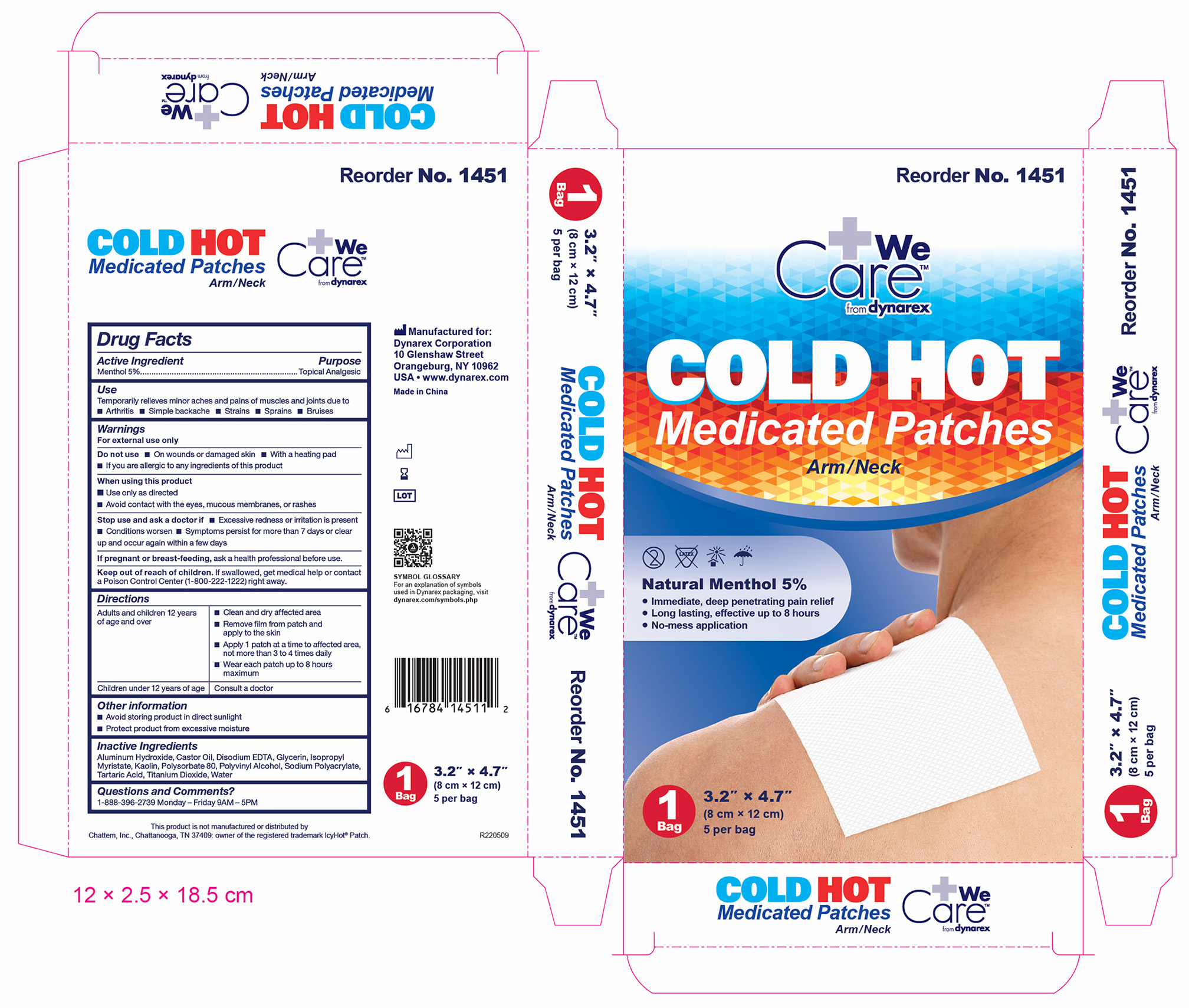
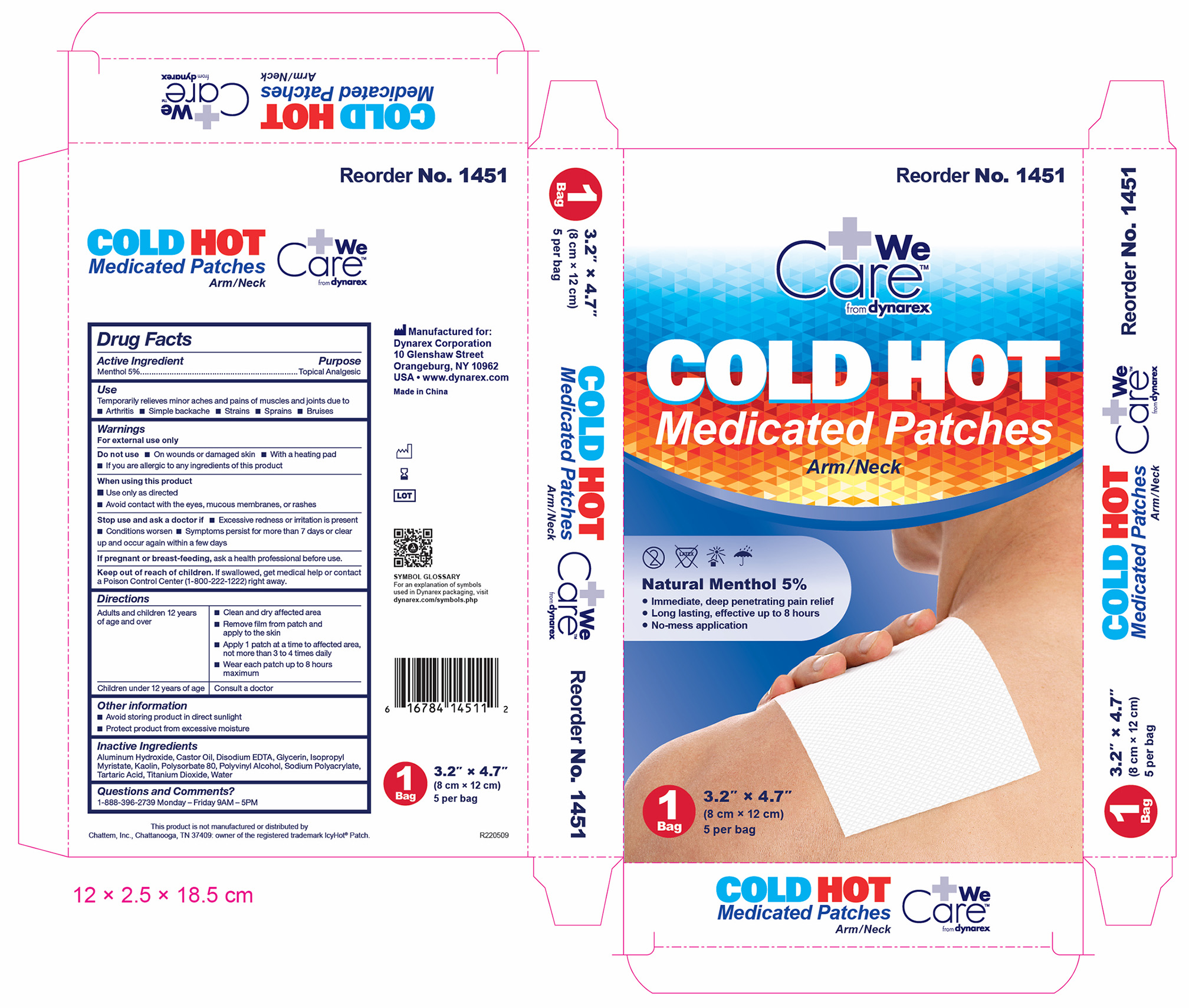 1451 Hot Cold Medicated Patch
1451 Hot Cold Medicated Patch
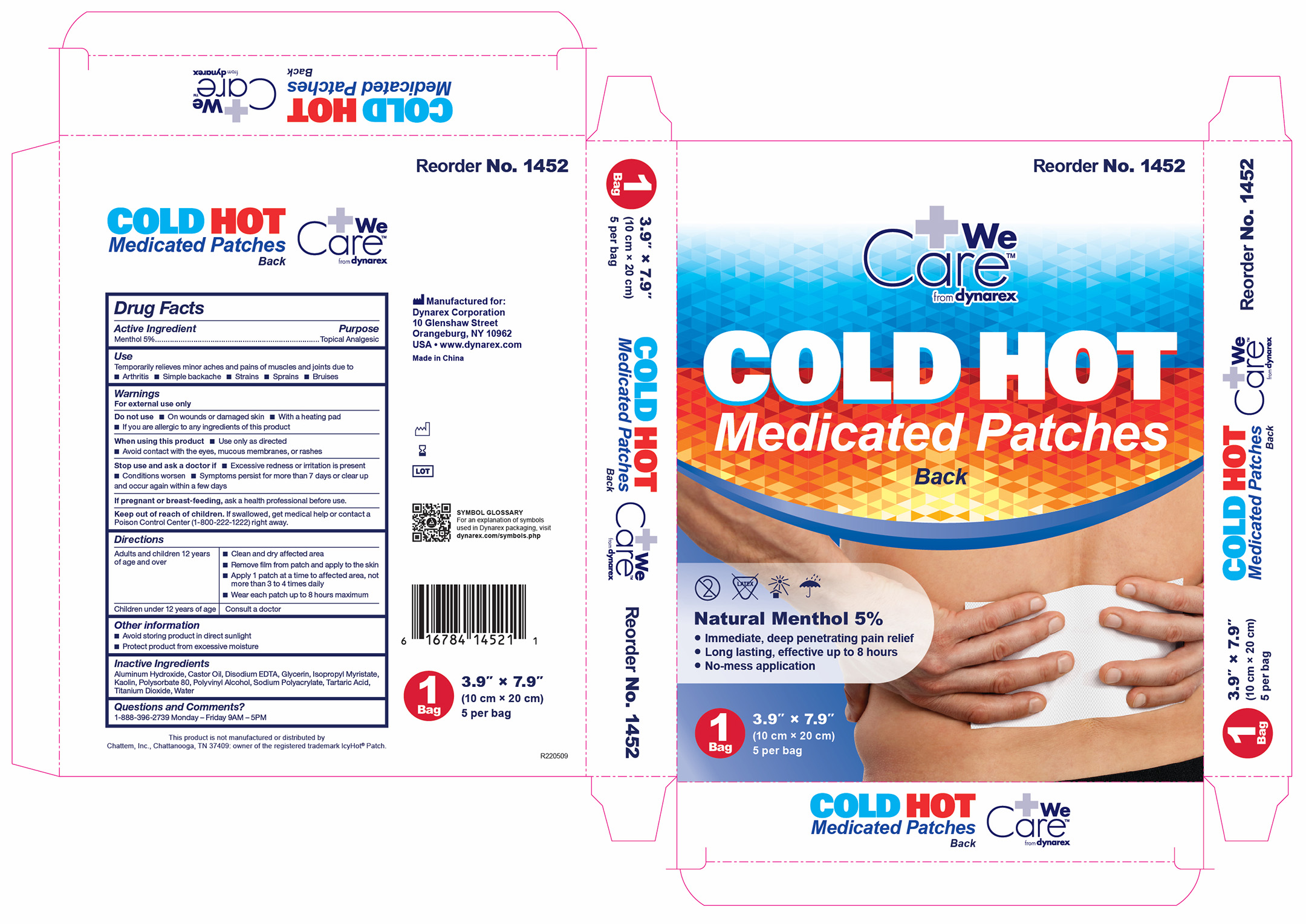
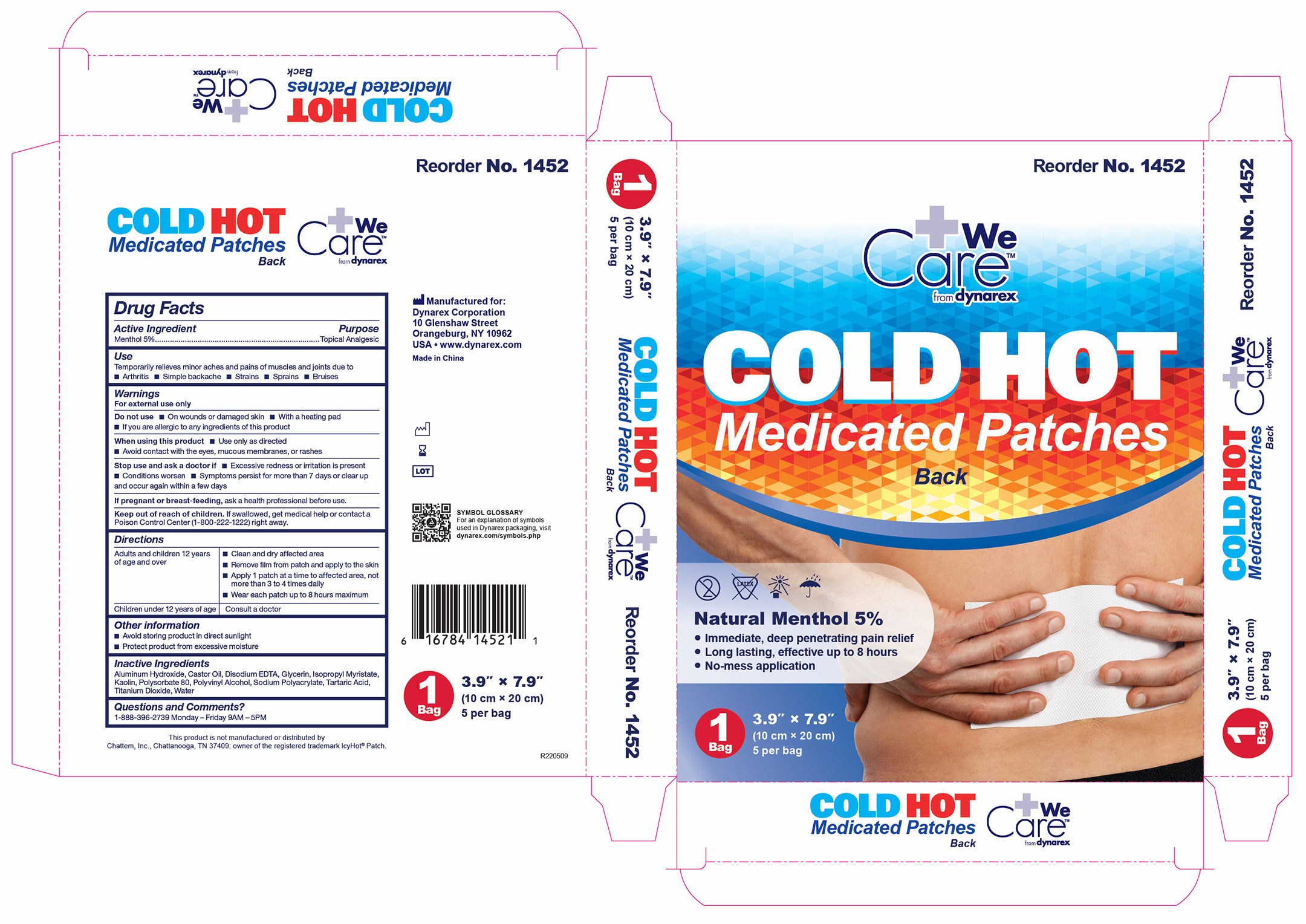 1452 Hot Cold Medicated Patch
1452 Hot Cold Medicated Patch