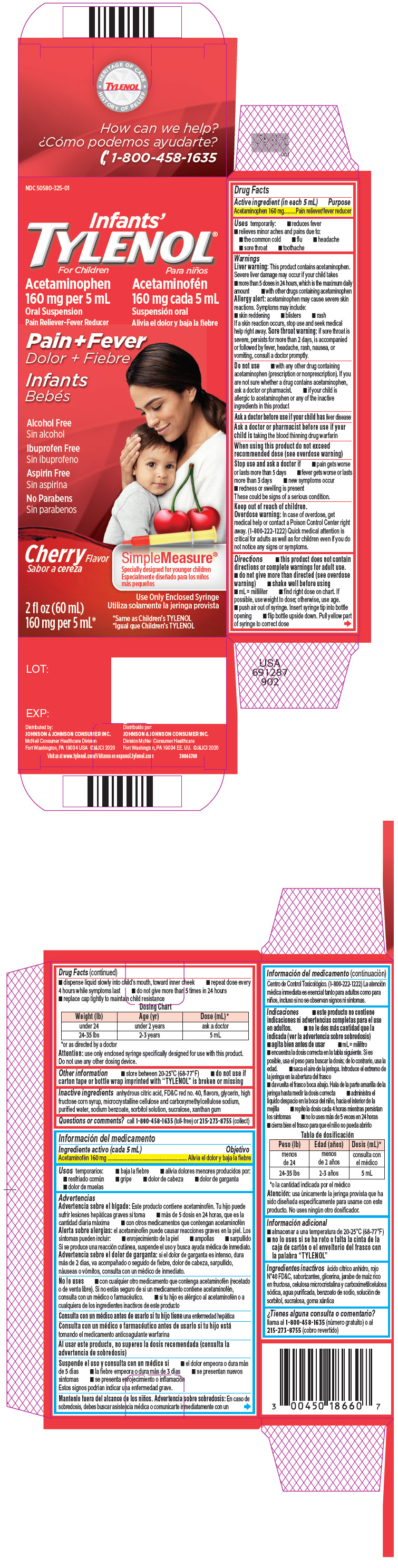
Label: INFANTS TYLENOL- acetaminophen suspension
- NDC Code(s): 50580-325-01
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 5 mL)
- Purpose
- Uses
-
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
When using this product do not exceed recommended dose (see overdose warning)
-
Directions
- this product does not contain directions or complete warnings for adult use.
- do not give more than directed (see overdose warning)
- shake well before using
- mL = milliliter
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
- push air out of syringe. Insert syringe tip into bottle opening
- flip bottle upside down. Pull yellow part of syringe to correct dose
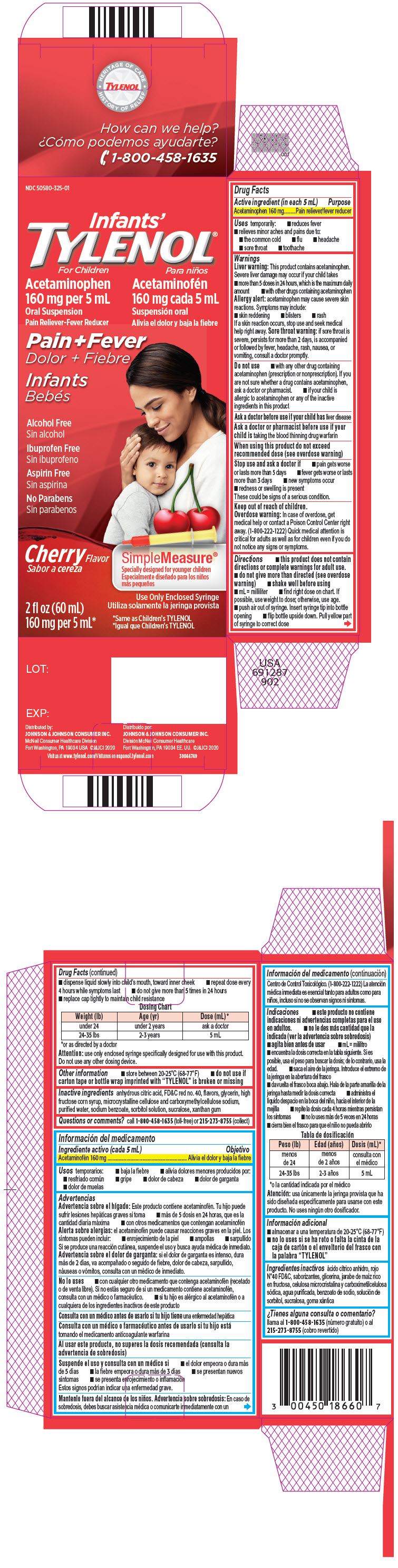
- dispense liquid slowly into child's mouth, toward inner cheek
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- replace cap tightly to maintain child resistance
Dosing Chart Weight (lb) Age (yr) Dose (mL) * - *
- or as directed by a doctor
under 24 under 2 years ask a doctor 24-35 lbs 2-3 years 5 mL Attention: use only enclosed syringe specifically designed for use with this product. Do not use any other dosing device.
- Other information
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL
NDC 50580-325-01
Infants'
TYLENOL ®
For ChildrenAcetaminophen
160 mg per 5 mL
Oral Suspension
Pain Reliever-Fever ReducerPain+Fever
Infants
Alcohol Free
Ibuprofen Free
Aspirin Free
No Parabens
Cherry Flavor
SimpleMeasure ®
Specially designed for younger children2 fl oz (60 mL)
160 mg per 5 mL*Use Only Enclosed Syringe
*Same as Children's TYLENOL
-
INGREDIENTS AND APPEARANCE
INFANTS TYLENOL
acetaminophen suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50580-325 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL SOLUTION (UNII: 8KW3E207O2) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50580-325-01 1 in 1 CARTON 09/25/2017 1 60 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 09/25/2017 Labeler - Johnson & Johnson Consumer Inc. (878046358)